Chemical modification of the backbone of synthetic oligonucleotides is commonly used for a variety of reasons: to increase the stability of the phosphodiester bond; to adjust duplex stability; to change the conformation of DNA or RNA; or to increase cellular uptake. Some of these modifications include phosphorothioates (Glen Report 18.1, and supplement in 2006), phosphorodithioates (Glen Report 20.1, 2008), and phosphonoacetates (Glen Report 20.2, 2008). Modification of the sugar moiety is commonly used for increasing nuclease resistance or increasing affinity of the oligo to its complementary target. The number of sugar modifications is increasing with the availability of more commercial monomers with non-natural sugars. The first commercially available sugar modification [introduced in the Glen catalogue in 1991] was the 2’-OMe analogue1, which improves the stability of DNA and RNA to nuclease degradation and increases the Tm of the target duplex.
More recently, locked nucleic acid (LNA) – also named bridged nucleic acid (BNA) in Japan – became quite popular for introducing modifications, for example, in probes and siRNA.2,3,4,5 Unfortunately, Glen Research is no longer able to offer LNA monomers and they are now only available directly from Exiqon.
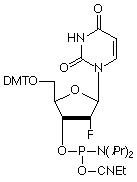
A search for a suitable alternative that could offer some of the same properties as the LNA monomers led us to consider the currently available 2’-fluoro RNA monomers. First of all, the introduction of 2’-F-RNA residues into synthetic oligonucleotides increases the Tm of the duplex by about 1 to 2 °C per incorporation.6 Also oligonucleotides containing 2’-F residues are resistant to enzymatic degradation7 and siRNAs modified with 2’-F RNA are more resistant to enzymatic degradation than unmodified siRNAs, both in vitro and in vivo.8 It is also known9 that 2’-F nucleotides prefer a C3’-endo/north conformation while LNA nucleotides contain a covalent linkage that restricts pseudorotation of the ribose to the C3’-endo conformation. 4,10
Oligonucleotides containing 2’-F RNA residues have been used as antisense11,12 and as siRNA8,13 oligonucleotides. RNA oligonucleotides can even be completely substituted with alternating 2' modifications (2'-OMe and 2'-F) and retain the ability to silence gene expression in human cells, even exhibiting up to a 500 fold increase in potency relative to native siRNA.14
Also 2’-F nucleotides proved to be active and beneficial in aptamers15 as well as in antagomirs16. These synthetic oligos are able to silence micro-RNAs in vitro and in vivo.
One of the biggest advantages of 2’-F RNA is that the monomers are commercially available at a significant savings over LNA monomers. We are delighted to be able to offer 2'-F-RNA monomers at prices which have been significantly lowered.
While LNA monomers and oligos are protected by several patents filed by Danish and Japanese groups, our customers should also be aware that a license may be required from Isis Pharmaceuticals, Inc. to incorporate 2’-F modified nucleotides into oligonucleotides as claimed in US Patent Numbers 5,670,633; 6,005,087; 6,531,584 and foreign equivalents.