Click chemistry has gained such great fame that it now has its own entry in Wikipedia. This conjugation technique using 1,3-dipolar cyclo addition between an azide and a terminal alkyne was first discovered by Rolf Huisgen’s group at the University of Munich in the late 50’s.1 Later, the term "Click Chemistry" was introduced for the simple, efficient labeling of biomolecules such as DNA, RNA or peptides by Sharpless, whose significant contribution was the addition of copper iodide as a catalyst to dramatically increase the rate of cycloaddition of the azide to the alkyne. 2 In general terms, this cycloaddition can be summarized as shown in Figure 1.



In previous Glen Reports [GR19.1 (2007) and GR20.1 (2008)], Glen Research introduced 5’-Hexynyl Phosphoramidite [10-1908], Azidobutyrate NHS Ester [50-1904], and 5’-Bromohexyl Phosphoramidite [10-1946], for use in in cycloaddition reactions. With this brief article, we introduce a new set of products for this application.
NHS esters are common and robust reagents for labeling DNA, RNA and other biomolecules. The popularity of our Azidobutyrate-NHS ester has demonstrated this fact yet again. Consequently, we now introduce Alkyne NHS ester [50-1905] to allow the functionalization of an amino moiety in a variety of molecules, including DNA and RNA oligonucleotides as well as peptides or proteins. In DNA or RNA this could be at the 5’-terminus, 3’-terminus, or intramolecular for oligos modified using any of our amino modifiers. This allows researchers to use a variety of products from the Click Chemistry library that are capable of reacting with NHS esters.
After customer requests to offer additional choices for Click Chemistry, we are pleased to offer a synthesis support for labelling the 3’ terminus of oligonucleotides with an alkyne group for use in Click Chemistry. This builds upon our 1,3-diol product portfolio with the serinol backbone introduced in our Glen Report in 2008 (GR20.2).
We expect that these additions to our Click product portfolio will become popular tools. Our commitment to Click Chemistry remains strong and we look forward to further additions in future Glen Reports.
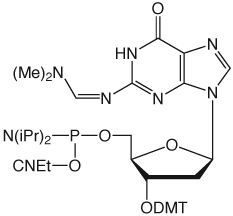
We are supplementing our range of products for 5'->3' synthesis by adding dmf-dG-5'-CE Phosphoramidite. Using the dimethylformamidine (dmf) protecting group is a clear improvement over the use of isobutyryl (ibu) for dG since dmf is removed around 4X as quickly as ibu. This is especially important for the synthesis of oligos containing labile minor bases or fluorescent tags. Dmf-dG-5'-CE Phosphoramidite (10-9201) is offered at the same price as the original ibu-dG product.