Crosslinking is used to stabilize the interaction of two weakly bound biomolecules using covalent bonds. Examples of the use of crosslinking include affinity labeling of DNA- and RNA-binding proteins, inter-strand crosslinks between DNA and/or RNA, and capturing any molecule’s approach to DNA or RNA. Crosslinking may be initiated by a variety of chemical reactions, for example, disulfide formation, but the predominant procedure is photo-induced crosslinking.
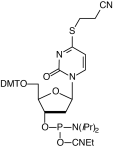
Ligation may be considered to be a subset of crosslinking. In non-enzymatic ligation of oligonucleotides, the 5'-terminus of the oligonucleotide to be ligated is modified as a 5'-iodo group. The 3'-terminus to be ligated is modified as a 3'-thiophosphate, prepared by sulfurizing in the first cycle using 3'-phosphate CPG. Chemical ligation occurs spontaneously by reaction of the thiophosphate to displace the iodo group using a short "splint" oligonucleotide complementary to the ends to bring them together.
A possible alternative approach to the chemical synthesis of large DNA strands is to join together synthetic oligonucleotides by chemical methods. Recently click ligation by the copper-catalyzed azide-alkyne cycloaddition (CuAAC) has been shown to facilitate this process, and a biocompatible triazole linkage has been developed that mimics a normal phosphodiester group. This requires an oligonucleotide with a 5’-azide and another with a 3’-propargyl group. The two oligonucleotides can be joined together by splint mediated ligation to produce a triazole linkage at the ligation site.


5'-O-(4,4'-Dimethoxytrityl)-1'-(3-cyanovinylcarbazol-9-yl)-2'-deoxy-β-D-ribofuranosyl- 3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite