Cellular DNA is constantly being damaged by oxidation and alkylation, by free radicals, and by ultraviolet and ionizing radiation. The body has therefore evolved a number of repair enzyme systems to excise and repair these lesions. The 8-oxo purine monomers allow investigation of the structure and activity of oligonucleotides containing an 8-oxo mutation, which is formed naturally when DNA is subjected to oxidative conditions or ionizing radiation. 5,6-Dihydro pyrimidines are naturally occurring compounds that are structural components of alanine transfer RNA. Dihydrouracil and the hydroxy pyrimidines are major base damage products formed by exposure of DNA to ionizing radiation.
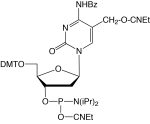
8-Amino-dG is formed along with 8-oxo-dG as the major mutagenic lesions formed in DNA damage caused by 2-nitropropane. 2-Nitropropane is an industrial solvent, a component of paints, dyes and varnishes, and is also present in cigarette smoke. Thymine glycol (5,6-dihydroxy-5,6-dihydrothymine) is formed when thymine is subjected to oxidative stress, including ionizing radiation. Oxidation of the 5,6 double bond of Thymidine generates two chiral centers at C5 and C6. The cis-5R,6S form is generated as the predominant product along with the other diastereomer, the cis-5S,6R form. The presence of thymidine glycol in DNA has significant biological consequences, and many organisms possess specific repair enzymes for the excision of this lesion. Hydrolysis of nucleoside residues in DNA occurs to generate abasic sites. Most commonly, dA sites are hydrolyzed causing depurination and leading to abasic residues. For researchers trying to determine if their source of depurination in chemical synthesis of DNA is reagent, fluidics or protocol-based, we offer a depurination-resistant dA monomer. A new chemical method allows the generation of abasic sites in double and single stranded oligonucleotides using very mild specific conditions and with very low probability of side reactions. The original Abasic Phosphoramidite (10-1924) has been discontinued since it exhibits low coupling efficiency and the post-synthesis chemistry is fairly challenging. Abasic II Phosphoramidite1 is the replacement for the preparation of a true abasic site. This product has the advantage of simplicity in that the silyl group is removed post-synthesis using aqueous acetic acid. dSpacer has also been used successfully as a mimic of the highly base-labile abasic site.
One of the major sources of DNA damage in all organisms is the UV component of sunlight. The predominant reaction induced by UV light on DNA is dimerization of adjacent pyrimidine bases leading to cyclobutane dimers (CPDs). The dimers formed in the most significant quantity are the cis-syn cyclobutane dimer of two thymine bases. Although formed routinely, these dimer products are efficiently excised and repaired enzymatically by nucleotide excision repair (NER) or the dimerization is reversed by photolyase enzymes. A further mode of oxidative damage is radiation-induced damage of DNA, which has been shown to lead to bridged cyclonucleosides. The purines, cyclo-dA and cyclo-dG, are predominantly formed, although the cyclo pyrimidines have also been detected. Cyclo-dA is doubly intriguing since it contains both damaged base and damaged sugar residues and, as such, should have a considerable biological impact. In a manner analogous to thymine dimer, cyclo purines cause significant distortion of the regular DNA helix and these lesions are repaired not by base excision repair (BER) but by NER.
Base excision repair (BER) is one of the most studied repair mechanisms. In this pathway, DNA glycosylases recognize the damaged bases and catalyze their excision through hydrolysis of the N-glycosidic bond. Attempts to understand the structural basis for DNA damage recognition by DNA glycosylases have been hampered by the short-lived association of these enzymes with their DNA substrates. To overcome this problem, the Verdine group at Harvard synthesized a pyrrolidine analog that mimics the charged transition state of the enzyme-substrate complex. When incorporated into double-stranded DNA, they found the pyrrolidine analog (PYR), introduced as the Pyrrolidine-CE Phosphoramidite, forms an extremely stable complex with the DNA glycosylase AlkA, exhibiting a dissociation constant in the pM range and potently inhibiting the reaction catalyzed by the enzyme.