Glen Report 21.16b: New Products - CPR II CPG
CPR II CPG
3'-Phosphate CPG, Catalog No.: 20-2900, (1) in Figure 2, has proved to be a popular and successful product for the preparation of oligonucleotide-3'-phosphates. However, the sulfonylethyl group in this molecule is susceptible to ß-elimination, precluding the ability to conduct base-mediated reactions on the solid support. For example, many researchers treat synthesis supports with a hindered base (e.g., diethylamine, diisopropylethylamine, or DBU) post-synthesis to eliminate and remove the cyanoethyl phosphate groups. In this way, the acrylonitrile formed in situ is removed from the support and is not available to alkylate dT residues at the N3 position in the oligos. Since the sulfonylethyl group in 3'-Phosphate CPG is also susceptible to ß-elimination leading to oligo cleavage, this technique is not compatible with 3'-phosphate CPG.
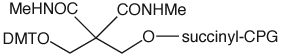
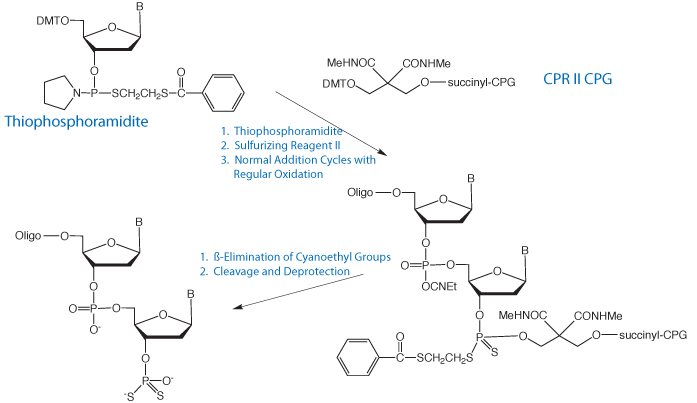
A more critical example where 3'-phosphate CPG is not compatible is the preparation of 3'-dithiophosphate oligos. These are formed using a phosphate CPG and a thiophosphoramidite in the first cycle. The dithioate is formed by sulfurization using Sulfurizing Reagent II. The synthesis of the oligo is then completed using regular cyanoethyl phosphoramidites. Sulfur is much more susceptible to alkylation by acrylonitrile and regular deprotection would lead to significant alkylation of the 3'-dithioate. Using CPR II CPG, (2) in Figure 2, which is base labile but does not support ß-elimination, the cyanoethyl groups can be removed from the oligo prior to cleavage and base deprotection. This process is illustrated in Figure 3.
We are happy to offer CPR II CPG, Catalog No.: 20-2903, to complement our offering of 3'-Phosphate CPG.

Ordering Information
- Glen Report 21.11: Hot Start PCR update: CleanAmp™ Primers
- Glen Report 21.12: Procedure for Synthesis of CleanAmp™ Primers
- Glen Report 21.13: New Product - (5‘S)-5’,8-Cyclo-dA CE Phosphoramidite
- Glen Report 21.14: Technical Brief - Preparation of Oligonucleotides Containing Abasic Sites
- Glen Report 21.15: Deprotection - Volume 2 - RNA Deprotection
- Glen Report 21.16a: New Products - High Load Glen UnySupport
- Glen Report 21.16b: New Products - CPR II CPG
- Glen Report 21.17: New Products – Click Chemistry, DMF-dG-5'-CE Phosphoramidite
- Glen Report 21.18: Technical Brief – LNA vs 2’-F-RNA
- Glen Report 21.19: Technical Brief – Avoiding Trityl Loss When Processing Synthetic Oligonucleotides
- Glen Report 21.110: New Fluorescent Phosphoramidites - SIMA (HEX), DyLight™

