A major issue with the processing of oligonucleotides, especially for large-scale synthesis, is the loss of the 5’-trityl group when drying down an oligonucleotide in preparation for a trityl-on purification or down-stream processing. Even on a small scale, oligonucleotides can lose 5’-trityl protection upon drying – but the amount of trityl loss can be variable.
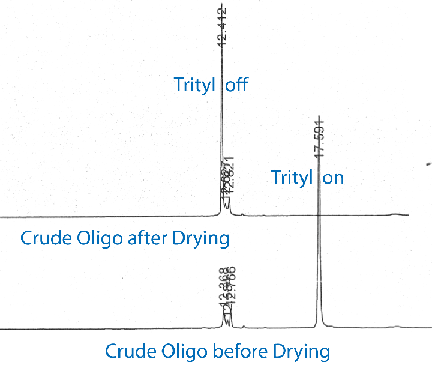
This trityl loss can be especially prominent for trityl-protected amino-modifiers, such as 5’-Amino-Modifier C6 (10-1906) and the latest version, 5’-DMS(O)MT Amino-Modifier C6 (10-1907). The chromatograms for a 5’-DMS(O)MT Amino-Modified oligo before and after drying, shown in Figure 1, demonstrate the complete loss of the trityl protecting group on drying.
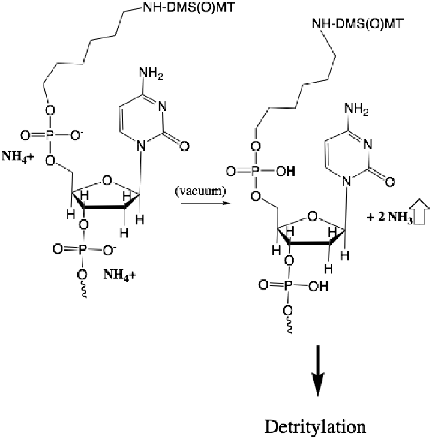
One observation we noted was that drying for extended periods of time, or the use of very high vacuum when drying down the oligonucleotides, resulted in greater amounts of trityl loss. This led us to the hypothesis that high vacuum was driving the equilibrium from a protonated volatile base as a counterion toward the nucleic acid with loss of either ammonia or methylamine gas, as shown in Figure 2.
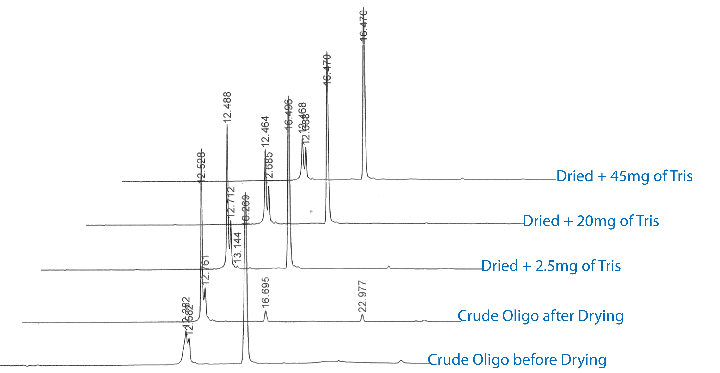
If this model is indeed correct, a simple test would be to dry the oligo down in the presence of varying amounts of a non-volatile base such as Tris. The results of these experiments, shown in Figure 3, indicate that the addition of Tris prevented the loss of the 5’-trityl in a concentration-dependent manner. Addition of 45mg of Tris base/mL of crude oligo solution prevented any loss of the trityl group.
The data noted above prompted us to revisit our recommended deprotection conditions for both the 5’-DMS(O)MT Amino-Modifer C6 and the MMT-protected 5’-Amino-Modifier C6. For these products, we have always recommended deprotection at 40°C or lower to avoid reported thermally-induced trityl loss. We deprotected trityl-amino-modified oligos in three ways: room temperature ammonium hydroxide, ammonium hydroxide for 17 hr at 55°C, and finally deprotection in AMA at 65°C for 10 minutes. HPLC analysis revealed that there was no indication of any loss of the trityl protecting group during any of the deprotection conditions at elevated temperatures. We are now able to remove the recommendation that these amino-modified oligos should be deprotected at 40°C or lower.
The addition of large amounts of salt can be problematic for downstream applications. A good example of this would be the synthesis of a 5’-amino-modified RNA in which the 5’-trityl is maintained for trityl-on purification. While the addition of the Tris would maintain the trityl, its presence would severely inhibit the removal of the 2’-silyl protecting groups in either TEA.3HF or TBAF. Therefore, we tested the possibility of converting the oligonucleotide to the nonvolatile sodium salt to maintain the trityl upon drying. The following desalting protocol was used on the model oligo 5’-DMS(O)MT Amino-Modifier T12:
HPLC analysis revealed that the crude amino-modified oligo without drying, the Glen-Pak purified oligo converted to the sodium salt prior to drying, and finally the eluted and dried oligo were identical in purity and that the trityl group was maintained throughout the process.