Glen Report 21.13: New Product - (5‘S)-5’,8-Cyclo-dA CE Phosphoramidite
Cellular DNA damage is predominantly due to the action of the short wavelength ultraviolet (UV) component of sunlight on skin cells and the result of oxidation by free radicals generated during routine metabolic activities. Such interactions with individual nucleotides can lead to mutations that are linked to carcinogenesis, neurodegeneration and general aging. These are compelling reasons indeed for investigation.
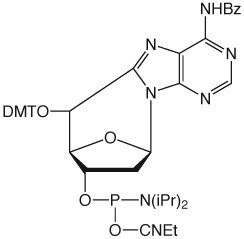
5',8-Cyclo-dA
Nucleotide excision repair (NER) is the principle mechanism for repair of DNA damage induced by sunlight and is also involved in the repair of free radical induced damage. The formation of thymine dimer is the most common DNA lesion from the UV component of sunlight. Thymine dimer phosphoramidite is available from Glen Research, Catalog No.: 11-1330. The NER mechanism is likely to be invoked for repair of lesions that significantly distort the DNA helical structure. The most common studies of oxidative DNA damage have revolved around the damaged nucleosides 8-oxo-dG and thymidine glycol, both of which are available as phosphoramidite derivatives, Catalog Nos.: 10-1028 and 10-1096, respectively. Oxidative DNA damage is more likely to be repaired by base excision repair (BER). Indeed, BER has been shown to be the main repair pathway for the lesions 8-oxo-dG and thymidine glycol.1,2
A further mode of oxidative damage is radiation-induced damage of DNA, which has been shown to lead to bridged cyclonucleosides. The purines, cyclo-dA and cyclo-dG, are predominantly formed, although the cyclo pyrimidines have also been detected. Cyclo-dA is formed when a free radical is induced at the C5’ of the deoxyribose sugar by attack by hydroxyl radicals, photolysis or other ionizing processes. The C5’ free radical inserts into the C8-N7 double bond of the adjacent adenine residue to form 5’,8-cyclo-dA as either a 5’R or 5’S diastereomer. Of the two diastereomers, the (5’S) cyclo-dA appears to be the most cytotoxic.3 Cyclo-dA predominates over cyclo-dG in DNA damage. In a manner analogous to thymine dimer, cyclo purines cause significant distortion of the regular DNA helix and these lesions are repaired not by BER but by NER.4,5
The NER proteins target base lesions which severely distort the helix, such as cyclo-dA and thymidine dimer. In the absence of repair by NER proteins, cyclo-dA is a strong block to both transcription and replication. Interestingly, there are low fidelity polymerases in the Y family that allow the genome to be copied even in the presence of bulky base lesions.6 These polymerases are responsible for the build up of tolerance for anticancer cis-platin drugs that crosslink neighboring guanosine residues. Over time, the tumor is enriched in cells that overproduce these polymerases, allowing its survival.7 Marietta and Brooks found that cyclo-dA and thymine dimer are strong but incomplete blocks to Pol II transcription in NER-deficient human cell lines.8 These transcripts, however, often contained multiple nucleotide deletions. Their results were consistent with the current models of transcription-coupled NER in which Cockayne syndrome proteins are recruited at the Pol II stall site, resulting in nucleotide excision repair of the lesion.9
Cyclo-dA is doubly intriguing since it contains both damaged base and damaged sugar residues and, as such, should have a considerable biological impact.10 It is clear that in-depth analysis of the conformational changes caused by the cyclo-dA lesion and their biological effects requires the availability of Cyclo-dA-CE Phosphoramidite, which we introduce in this article, Catalog No.: 10-1098.
(5‘S)-5’,8-Cyclo-dA-CE Phosphor-amidite, Figure 1, can be used in oligonucleotide synthesis with only minor changes to the standard protocol. A three minute coupling time for cyclo-dA has been shown to be optimal. However, the subsequent phosphoramidite coupling time has to be increased to six minutes since the 5’-OH once formed is a secondary hydroxyl and is conformationally restricted. Unusually for damaged bases, cleavage and deprotection of oligonucleotides containing cyclo-dA can be achieved using standard procedures.
We are indebted to Didier Gasparutto, CEA - CEN Grenoble, for kindly reviewing and commenting on this article.
References
- P.J. Brooks, et al., J. Biol. Chem., 2000, 275, 22355-62.
- K. Randerath, G.D. Zhou, R.L. Somers, J.H. Robbins, and P.J. Brooks, J. Biol. Chem., 2001, 276, 36051-7.
- I. Kuraoka, et al., J. Biol. Chem., 2001, 276, 49283-49288.
- P. Jaruga, J. Theruvathu, M. Dizdaroglu, and P.J. Brooks, Nucleic Acids Res, 2004, 32, e87.
- P. Jaruga, and M. Dizdaroglu, DNA Repair (Amst), 2008, 7, 1413-25.
- E.C. Friedberg, A.R. Lehmann, and R.P. Fuchs, Mol Cell, 2005, 18, 499-505.
- M.R. Albertella, A. Lau, and M.J. O’Connor, DNA Repair (Amst), 2005, 4, 583-93.
- C. Marietta, and P.J. Brooks, EMBO Rep, 2007, 8, 388-93.
- J.O. Andressoo, and J.H. Hoeijmakers, Mutat Res, 2005, 577, 179-94.
- A. Romieu, D. Gasparutto, D. Molko, and J. Cadet, J Org Chem, 1998, 63, 5245-5249.
Product Information
8,5'-Cyclo-dA CE Phosphoramidite (10-1098) has been discontinued.
- Glen Report 21.11: Hot Start PCR update: CleanAmp™ Primers
- Glen Report 21.12: Procedure for Synthesis of CleanAmp™ Primers
- Glen Report 21.13: New Product - (5‘S)-5’,8-Cyclo-dA CE Phosphoramidite
- Glen Report 21.14: Technical Brief - Preparation of Oligonucleotides Containing Abasic Sites
- Glen Report 21.15: Deprotection - Volume 2 - RNA Deprotection
- Glen Report 21.16a: New Products - High Load Glen UnySupport
- Glen Report 21.16b: New Products - CPR II CPG
- Glen Report 21.17: New Products – Click Chemistry, DMF-dG-5'-CE Phosphoramidite
- Glen Report 21.18: Technical Brief – LNA vs 2’-F-RNA
- Glen Report 21.19: Technical Brief – Avoiding Trityl Loss When Processing Synthetic Oligonucleotides
- Glen Report 21.110: New Fluorescent Phosphoramidites - SIMA (HEX), DyLight™

