A triumvirate of fluorescein derivatives has dominated fluorescent labelling of oligonucleotides over the last decade. Fluorescein, hexachlorofluorescein and tetrachlorofluorescein, better known as FAM, HEX and TET, have been in the forefront of DNA analysis in the fields of sequencing and genetic analysis using instruments with multicolor detection. However, HEX has proved to be the weakest performer of the three due to its instability to the basic conditions of oligonucleotide deprotection. HEX-labelled oligonucleotides must be treated with care to avoid some level of degradation. Moreover, the mechanism of degradation involves the loss of chlorine residues from the ring systems leading to products that are fluorescent at lower wavelengths, even covering the wavelength of TET.
In the previous Glen Report, we introduced a new fluorescein phosphoramidite, 5’- dichloro-dimethoxy fluorescein, better known as JOE™ in the world of multicolor sequencing. This fluorescein derivative exhibits excellent stability to a range of deprotection conditions. In addition, its fluorescence is much less pH sensitive than fluorescein itself.
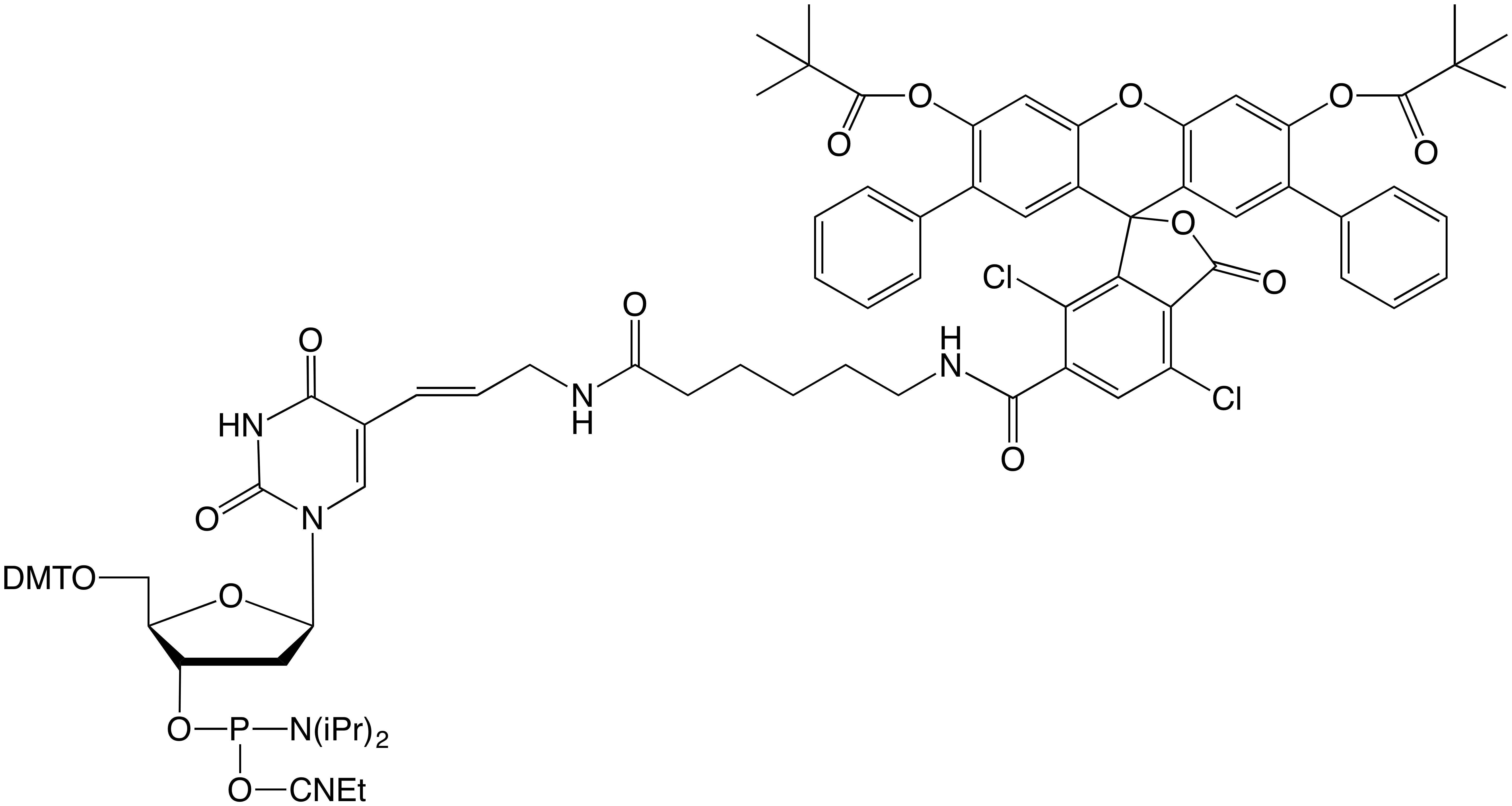
Now, we are happy to introduce further fluorescein-based products: SIMA (HEX) (1) and SIMA (HEX)-dT (2) in Figure 1, which exhibit virtually identical absorbance and emission spectra as HEX.
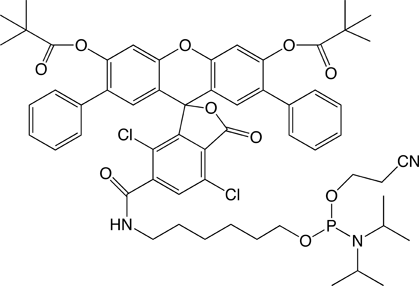
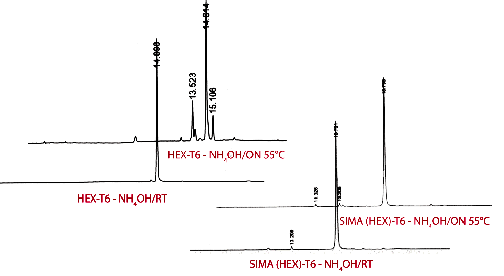
Dichloro-diphenyl-fluorescein, SIMA (HEX), is much more stable to basic deprotection conditions than HEX and oligonucleotides can be deprotected using ammonium hydroxide at elevated temperatures and even ammonium hydroxide/methylamine (AMA) at room temperature or 65°C for 10 minutes. The increased stability of SIMA (HEX) over HEX is illustrated in Figure 2. The HEX-labelled oligo deprotected with ammonium hydroxide at 55°C overnight shows considerable degradation, while the SIMA (HEX) equivalent shows no decomposition. Deprotection with AMA at 65°C for 10 minutes led to around 10% decomposition of HEX while SIMA (HEX) showed no signs of degradation. Similar levels of degradation of HEX and stability of SIMA (HEX) were found during deprotection with AMA at room temperature for 2 hours.
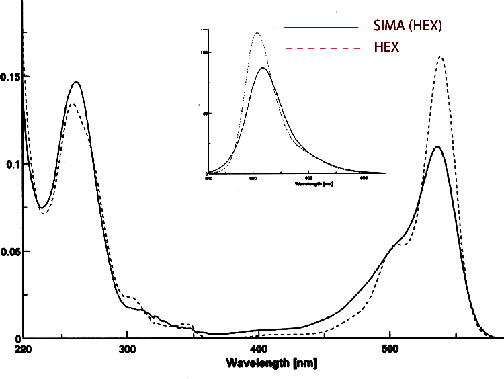
SIMA (HEX) exhibits absorption and emission maxima that are virtually identical to HEX, as shown in Figure 3. SIMA absorption maximum was 3 nm blue-shifted compared to HEX at pH 7. The absorbance is broader, so the extinction coefficient is smaller than that of HEX, but when exciting at 500 nm where the absorbance was normalized, the emission was still 90% of HEX and the emission was red-shifted by 5 nm.
The extinction coefficients of SIMA (HEX) determined at pH 7.0 are:
As an added bonus, SIMA (HEX) can be offered at significantly lower prices than HEX itself.
A second SIMA (HEX) product, SIMA (HEX)-dT, can be used to introduce SIMA (HEX) in the synthetic oligonucleotide sequence, usually as a replacement for the native dT linkage. Again, this product is fully compatible with deprotection schemes using ammonium hydroxide at elevated temperatures or AMA at room temperature and 65°C.
Cyanine dyes are highly fluorescent molecules that have become very popular in biological imaging. Several versions of cyanine dyes are available commercially with the most popular being the CyDye™ series from GE Healthcare. Cyanine 3, Cyanine 3.5, Cyanine 5 and Cyanine 5.5 phosphoramidites are already offered for sale by Glen Research under license from GE Healthcare. Commercial applications for Cy-labelled oligonucleotides are also covered by GE Healthcare's suite of patents. Consequently, a rapidly expanding market segment has opened up for generic, unpatented cyanine dyes that do not have the constraints of the licensing and royalty fees associated with Cyanine dyes.
We are happy to begin offering two DyLight™ dyes, DY547 and DY647, as phosphoramidite alternatives to Cyanine 3 and Cyanine 5. The structures of these two products are shown in Figure 4. The performance of these two dyes is similar to the equivalent Cy dyes and the absorption and emission spectra are virtually identical. This is demonstrated in Figure 5, which shows the absorption spectra of Cyanine 3 and DY547 oligos and the equivalent spectra for Cyanine 5 and DY647. Similarly, Figure 6 shows the fluorescence emission spectra of Cyanine 3 and DY547 oligos and the equivalent spectra for Cyanine 5 and DY 647.


A comparison of the emission or absorbance spectra of the oligos shown in Figures 5 and 6 demonstrates that the spectra are virtually identical for Cyanine 3 and DY547. Similarly, Cyanine 5 and DY 647 were virtually identical. When oligos containing DY547 were excited at 488 nm, the quantum yield (QY) was 12% greater than oligos containing Cyanine 3. When oligos containing DY647 were excited at 580 nm, the QY was 5% greater and the emission was blue-shifted by only 1 nm compared to Cyanine 5.


SIMA (HEX) and the DyLight™ dyes, DY547 and DY647, are not covered by patents and can be used for any purpose, including use by oligo synthesis companies and in vitro diagnostic development companies without need for a separate license.
DyLight is a trademark of Thermo Fisher Scientific Inc. and its subsidiaries.
SIMA (HEX) Phosphoramidite (10-5905)
SIMA (HEX)-dT Phosphoramidite (10-5945)
DyLight™ dyes have been discontinued.