2'-Deoxy-2’-fluoro-nucleosides adopt an RNA-type sugar conformation, presumably due to the high electronegativity of fluorine. Because of this sugar conformation, RNA duplexes (A-form) are generally more thermodynamically stable than DNA duplexes (B-form). As expected, the addition of 2’-F-RNA residues to oligodeoxynucleotides progressively increases the thermal stability of their duplexes with RNA. The stabilization is additive at approximately 2° per residue. This compares favorably with 2’-OMe-RNA at around 1.5° and RNA at 1.1° per residue. In the meantime, base pair specificity remains intact.
2’-F-RNA phosphodiester linkages are not nuclease resistant, although the corresponding phosphorothioate linkages are highly resistant. Researchers usually design antisense oligonucleotides to form duplexes with RNA, which are then substrates for RNase H. Uniformly modified 2’-F-RNA/RNA duplexes are not substrates for RNase H. However, it is straightforward to prepare chimeric 2’-F-RNA/DNA phosphorothioate oligonucleotides which exhibit enhanced binding to the RNA target, are substrates for RNase H, and are highly nuclease resistant.

5'-Dimethoxytrityl-N-benzoyl-deoxyAdenosine, 2'-fluoro-3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite

5'-Dimethoxytrityl-N-acetyl-deoxyCytidine,2'-fluoro-3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite

5'-Dimethoxytrityl-N-isobutyryl-deoxyGuanosine, 2'-fluoro-3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite

5'-Dimethoxytrityl-deoxyUridine, 2'-fluoro-3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite
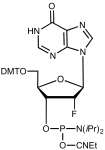
5'-Dimethoxytrityl-deoxyInosine, 2'-fluoro-3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite