Polyacrylamide gels have long since been essential tools in nucleic acid and peptide laboratories. The flexibility in preparing polyacrylamide gels allows unparalleled control of resolution through a broad range of sizes with a large loading capacity for the purification of nucleic acids. New applications for polyacrylamide gels continue to emerge.
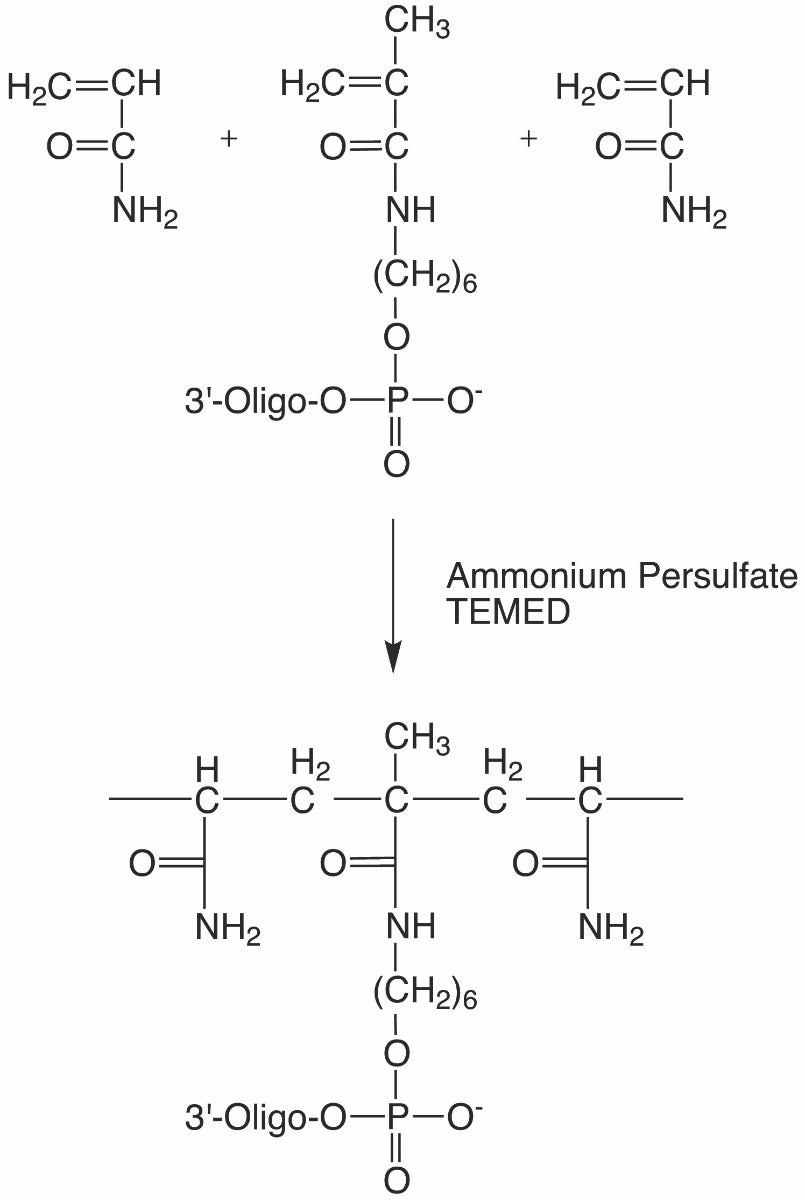
The copolymerization of acrylamide and bis-acrylamide crosslinker to form polyacrylamide gels is a vinyl addition polymerization reaction typically initiated by ammonium persulfate and tetramethylethylenediamine (TEMED). Ammonium persulfate acts as the free radical source and TEMED as a free radical catalyst. The persulfate free radicals react with acrylamide-containing monomers, converting them into additional free radicals, as well as polymerizing with unreacted acrylamide monomers to form the gel matrix. Including a 5’-methacrylate-labeled oligonucleotide in the reaction mixture covalently incorporates the oligonucleotide into the gel matrix.
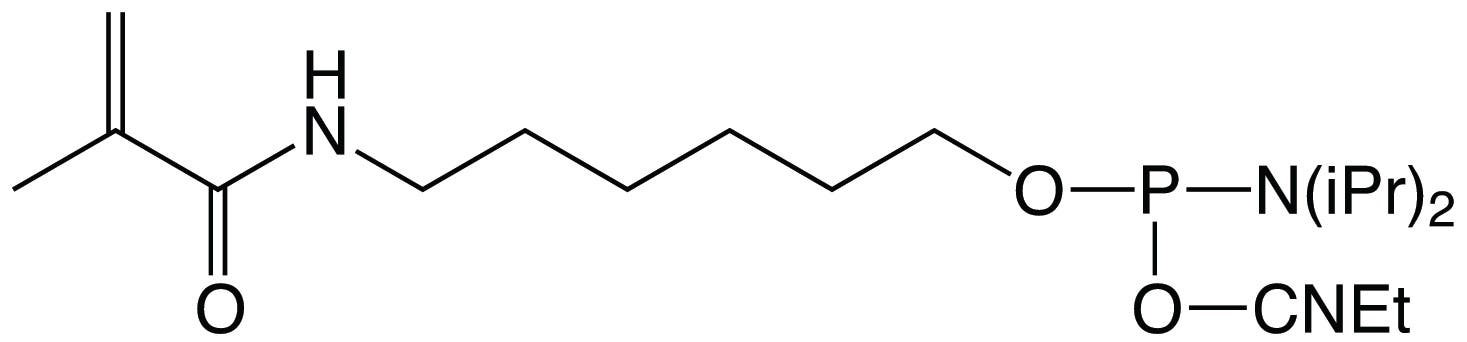
Methacrylate C6 Phosphoramidite (Figure 1) is a modifier that can be used to attach methacrylate to an oligonucleotide using conventional phosphoramidite chemistry and subsequently incorporated into a polymer (Figure 2). Methacrylate-modified oligonucleotides have been used to generate oligonucleotide-labeled hydrogels, microspheres, microarrays, and functionalized polymeric surfaces for use in purification, hybridization detection assays, affinity capture, biosensors, and sequencing.
In the first hybridization proof-of-concept experiments, methacrylate-labeled oligonucleotides were polymerized onto an acrylic-functionalized slide.1 Subsequent hybridization with asymmetric fluorescently-labeled PCR products confirmed the utility of acrylamide-labeled oligonucleotides in detection assays. This initial research on methacrylate-labeled oligonucleotides also confirmed that methacrylate labels are stable to standard PCR conditions and once polymerized, form polymers with high thermal stability, high density, and low non-specific absorption.1
More recently, methacrylate-labeled oligonucleotides have been used in aptamer-based hydrogels, molecular imprinting, and single molecule inexpensive FISH (smiFISH) techniques.2-4 Aptamer-based hydrogels use the selectivity of aptamers to bind their targets for detection using the physical properties of gels, as well as for electrochemical detection, and colorimetric detection. In one example, ochratoxin aptamers were incorporated into a hydrogel resulting in high selectivity for ochratoxin and a detection limit of 0.51 ppb in food products.5
Molecular imprinting uses biomolecules, such as antibodies, proteins, and glycoproteins, as templates to create molecularly imprinted polymers (MIPs).3 Biomolecules are mixed with the pre-polymer, polymerized in place, and subsequently removed to create a recognition cavity. Incorporating aptamers as the biomolecule in MIPs takes advantage of the binding affinity and selectivity of aptamers. As such, aptamer-based MIPs can enhance the sensitivity of MIPs down to femtomolar concentrations as shown for thrombin MIPs.3
In smFISH, a precursor to smiFISH, gene-specific primary fluorescent probes are used to localize individual mRNA within cells. By comparison, smiFISH probes are composed of primary and secondary probes. Primary probes are unlabeled and gene specific probes. Secondary probes are fluorescently labeled (FLAPs) and bind the FLAP sequence on the primary probes. When the secondary probe also contains a methacrylate label, smiFISH probes can be used in Expansion Microscopy (ExM).4,6,7 ExM is the in situ polymerization of labeled molecules followed by hydration of the matrix to induce expansion of the biological structure, retaining the 3D orientation of the original biological structure.6,7 Combining smiFISH with ExM provides a 2-fold increase in signal-to-noise ratio and the ability to resolve overlapping transcripts.4
In each of these techniques, methacrylate-labeled oligonucleotides are incorporated into a gel matrix and the canonical properties of the oligonucleotides provide specificity and functionality within the application. Glen Research is pleased to offer Methacrylate C6 Phosphoramidite for the synthesis of methacrylate-labeled oligonucleotides.
Methacrylate C6 Modifier is compatible with standard coupling times and deprotection conditions. This modifier does not contain a DMT protecting group and cannot be purified with DMT-ON purification techniques. However, the purification step can typically be omitted since only the oligonucleotides containing the methacrylate modification will be polymerized into the gel matrix. Unlabeled oligos are easily washed away after the polymerization step is completed.
1. F.N. Rehman, et al., Nucl Acid Res, 1999, 27, 649-655.
2. J. Liu, et al., Anal Bioanal Chem, 2012, 402, 187-94.
3. Z. Zhang and J. Liu, Small, (In Press), 201805246.
4. N. Tsanov, et al., Nucleic Acids Res, 2016, 44, e165.
5. R. Liu, et al., ACS Applied Materials & Interfaces, 2015, 7, 6982-6990.
6. F. Chen, P.W. Tillberg, and E.S. Boyden, Science (New York, N.Y.), 2015, 347, 543-548.
7. E.S. Boyden, 2016, http://bit.ly/2LgJz3d