Oligonucleotide synthesis typically proceeds from the 3’ to 5’ direction, mostly because the phosphoramidites for this direction are straight forward to synthesize. These reagents allow the synthesis of oligonucleotides for a majority of the required applications. However, there are a few scenarios where 5’ to 3’ synthesis reagents are necessary (Figures 1 and 2).
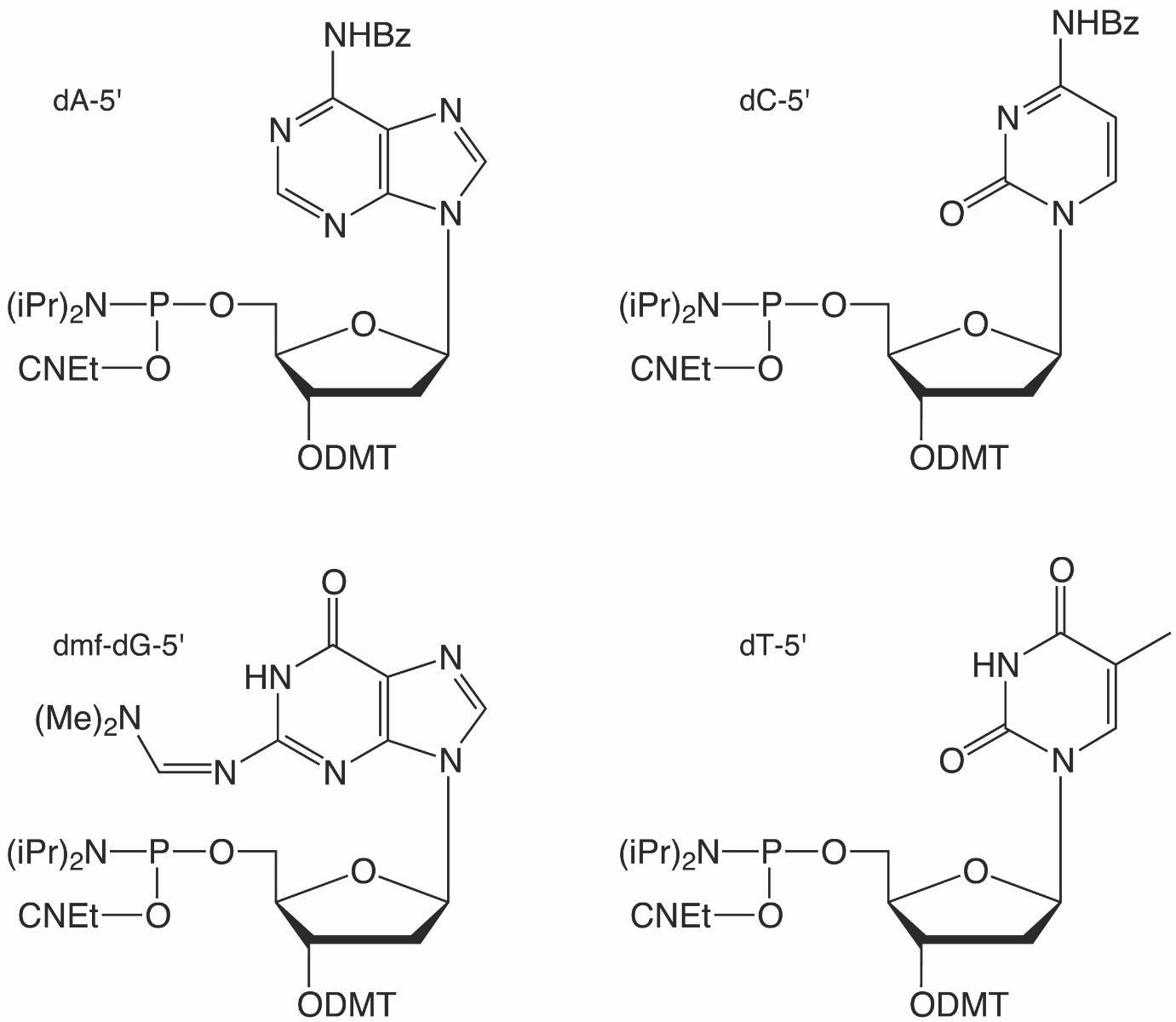
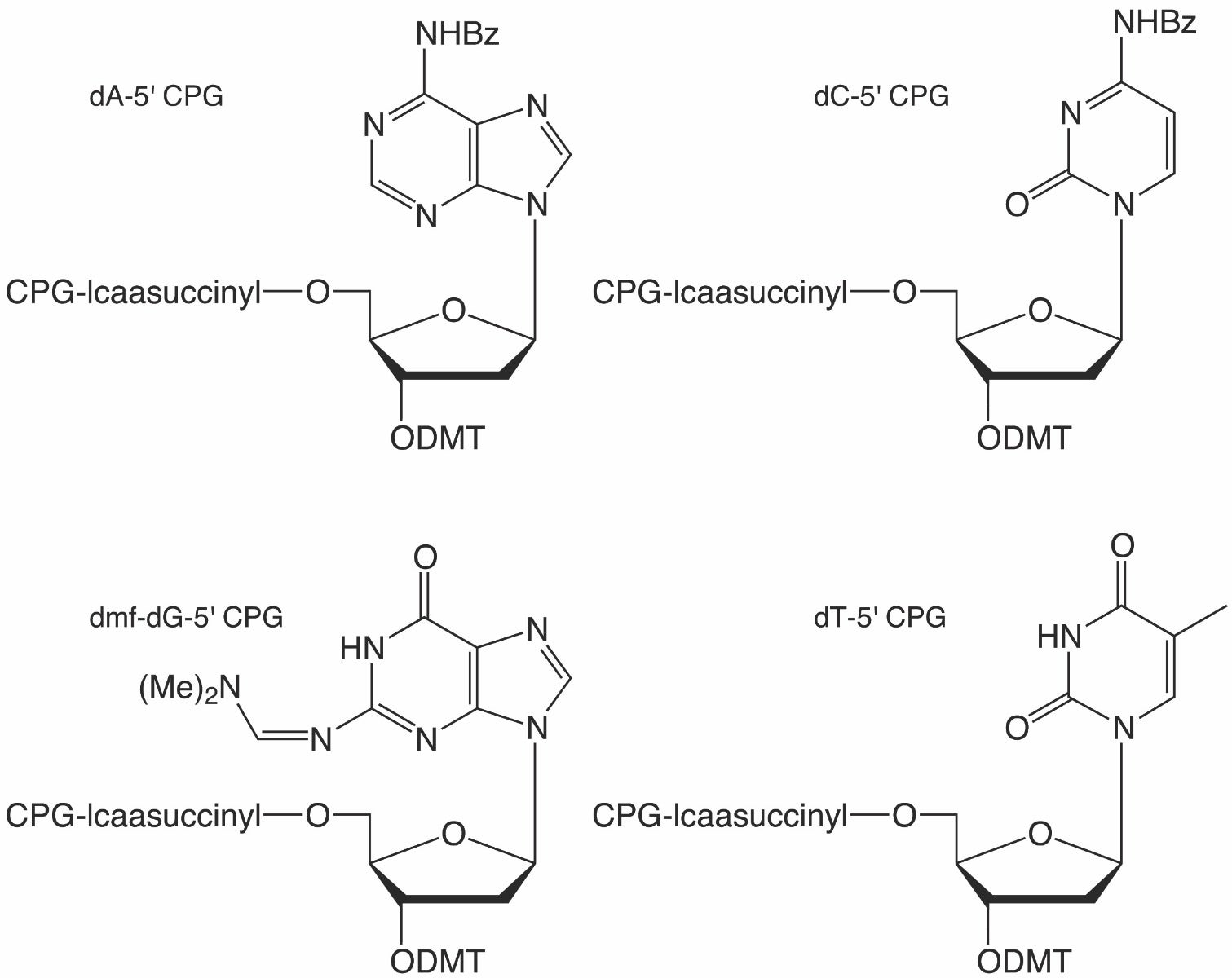
One of these scenarios is the generation of 3’ to 3’ linkages, particularly as a 3’-cap for nuclease resistance. Natural oligonucleotides are highly susceptible to nucleases, and the addition of an inverted nucleotide at the 3’-end dramatically increases resistance toward 3’-exonucleases.1 The most common method of introducing such a 3’-cap is to begin the synthesis with dT-5’-CPG (20-0302) and then carrying out the rest of the synthesis as one usually would in the 3’ to 5’ direction. The resulting oligonucleotide would have a 3’ inverted dT, and it should be noted that such a 3’-cap would also prevent polymerase extension.
A second scenario is the synthesis of oligonucleotides containing modifications at the 3’ terminus that would not be possible otherwise.2 Examples of such modifications include certain dideoxy nucleotides and phosphoramidite reagents that are only available as terminal labels.
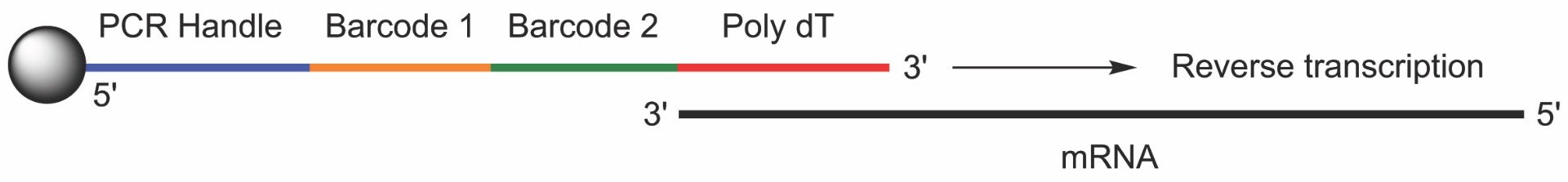
A third scenario is the synthesis of attached oligonucleotides that can be extended with polymerases. This type of synthesis arrangement is the key to single cell RNAseq methods such as Drop-seq3 that have been developed in recent years. In Drop-seq, the synthesis begins with mono-sized microparticles containing linkers that are stable to deprotection conditions thus ensuring the oligo remains attached to the bead (Figure 3). Briefly, reverse direction DNA synthesis is performed to synthesize relatively long oligonucleotides that contain several functional regions, one of which is a stretch of T’s. In a microfluidics environment, the poly T region of the oligonucleotide attached to one individual bead will hybridize to/capture the poly A tails of mRNA from one individual cell. A reverse transcriptase can then append a cDNA copy of the mRNA to the bead bound oligo. Each DNA sequence contains 2 barcode regions, one to differentiate one bead from another, and the other to differentiate one strand from another on the same bead. As a result, the DNA on these beads can be amplified by PCR for analysis by next generation sequencing to quantify individual mRNA sequences at the cellular level.
For many years now, Glen Research has been providing reverse DNA synthesis reagents to the research community. All these reagents have protecting groups and attachment points between the 5’ and 3’ positions that have been reversed. Everything else is identical; the protection groups are similar and the synthesizer protocols are the same.
Early on, Glen Research offered ibu-dG-5’-CE Phosphoramidite and ibu-dG-5’-CPG; however, several years ago, we began offering the dmf-protected version of the phosphoramidite in place of the ibu-protected version while leaving the CPG offering unchanged. Many customers who are performing reverse direction synthesis may not require the dG-CPG, such as Drop-seq researchers, but for those who require the dG-CPG as well, they are forced to use more harsh conditions necessary for removal of the ibu protecting group. As listed in the Glen Research Deprotection Guide, dmf-dG is significantly faster to deprotect when using standard deprotection conditions (Table 1). Due to this mismatch in protecting groups, Glen Research has added dmf-dG-5′-CPG to its catalog.
| Table 1. Deprotection of dG | ||
| dG Protection | Temperature | Time |
| ibu-dG | RT | 36h |
| 55°C | 16h | |
| 65°C | 8h | |
| dmf-dG | RT | 16h |
| 55°C | 4h | |
| 65°C | 2h | |