Gabriel De Crozals and Carole Chaix
Institut des Sciences Analytiques
Département LSA, équipe SIMS, UMR 5280, CNRS
Université Lyon 1
5, rue de la Doua, 69100 Villeurbanne Cedex
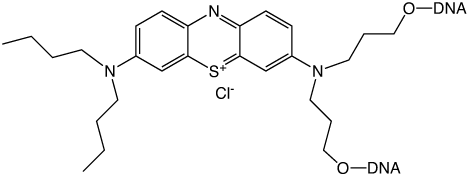
Dimethylaminophenothiazin-5-ium chloride, better known as Methylene Blue (MB), has many properties in chemistry and biology. For decades, this compound has been used for many applications, including redox indicator,1 photosensitizer,2-8 dye for cellular staining procedures,9 antiseptic2,10 and in medicine against Alzheimer's disease.11
For the detection of biological analytes, MB was used by many groups as a redox reporter bound to DNA probes.12-15 For instance, several studies were conducted by K. Plaxco et al. in the design of DNA, protein, antibody and drug biosensors.16-20 MB provided efficient electron transfer, good sensitivity and stability in such detection systems, and proved better than ferrocene.21
The position of MB along the DNA probe seems to be critical for sensor performance. For instance, internally labelled probes have shown better signal suppression after target addition than end-labelled probes.13 In this context, Barton's group studied the electrochemical response of MB bound to dsDNA with a flexible linker and demonstrated both direct reduction at the electrode and DNA-mediated reduction dependent on the π-stacking of MB.22,23
Intercalation properties of MB with DNA have been widely investigated but only with free MB molecules in solution.24-28 DNA structures with an intercalated MB still have to be considered, and novel binding chemistries have to be explored. However, only a post-synthesis functionalization of DNA with an NHS-ester derivative has been used to incorporate MB into oligonucleotides.22,29,30 This method is limited by the requirement for amino functions on the oligonucleotide chain. Although the introduction of amino groups is relatively straightforward with the appropriate phosphoramidites, the procedure requires an additional deprotection step followed by an additional coupling/purification procedure to incorporate the MB. This final step could be tedious due to the trend of MB to interact with DNA via electrostatic and π-stacking interactions with the phosphate backbone and nucleic bases (in particular with guanine residues), respectively, thus decreasing the efficiency of covalent incorporation.31,32
Our approach is to synthesize MB derivatives allowing covalent introduction into the desired position of an oligonucleotide sequence without supplementary manipulation. Phosphoramidite chemistry seems the most straightforward way to achieve this purpose. While most fluorescent dyes (fluorescein, rhodamine, cyanine, etc.) are now available as phosphoramidites, there is a lack of redox markers which can be incorporated during oligonucleotide solid-phase synthesis. For example, we can reference our work with ferrocene phosphoramidite that was shown to be efficient to incorporate numerous redox markers into sequences.33,34
Since many dyes are unstable under strong basic conditions, labelled DNA may have to be deprotected under mild basic conditions to ensure their integrity. It is worth noting that MB is not stable in these mild basic conditions and the molecule needs to be chemically modified to improve its stability.
We report the first synthesis of an MB phosphoramidite and its use for labelling synthetic DNA. First, a series of symmetrical MB analogues were synthesized and stability tests were performed in DNA synthesis and under DNA deprotection conditions. Second, after selection of the most stable molecule, unsymmetrical derivatives were obtained and modified as phosphoramidites.35
See also: New Product - Methylene Blue C3 Phosphoramidite
Methylene Blue C3 Phosphoramidite has been discontinued. Please see:
Glen Report 25.21 - Methylene Blue Phosphoramidite for DNA Labelling