Glen Report 25.24: New Products - TEMPO Spin Labels for Click Chemistry and 5-Ethynyl-dU
Spin labels are molecules containing sites with unpaired electrons, usually stable nitroxide free radicals, and generate a paramagnetic center that can be probed using magnetic resonance spectroscopy. Electron Paramagnetic Resonance (EPR) spectroscopy is a sensitive technique for the characterization of materials and has been used to determine the molecular environment of spin labels1,2, including the location, orientation and motion of spin labels within biomolecules. Most naturally occurring biomolecules do not contain unpaired electrons. Consequently, spin labels localized within biomolecules can act as bioorthogonal chemical reporters for studying the structure and dynamics of biomolecules using EPR spectroscopy.
Recently, the spin label 2,2,6,6- tetramethylpiperidine 1-oxyl (TEMPO) has been used to determine RNA structure using paramagnetic relaxation enhancement (PRE).3
Spin labels can be easily incorporated into biomolecules by covalent attachment using Click Chemistry. The wide variety of alkyne modifications already available from Glen Research offers a diverse set of options for site-specific conjugation of spin labels to oligonucleotides.
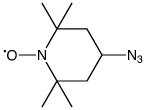
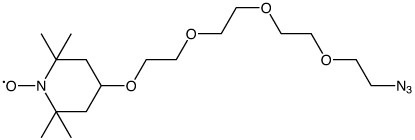
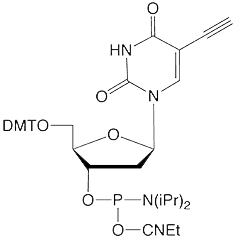
As shown in Figure 1, we offer two new nitroxide spin labels, TEMPO Azide (1) and TEMPO-TEG Azide (2), compatible with Click Chemistry for site directed spin labelling (SDSL). TEMPO Azide (1) is most suited for conjugation to an alkyne group rigidly attached to the biomolecule in question. To facilitate this situation in oligonucleotides, we are also introducing 5-Ethynyl-dU (3), for convenient click conjugation with TEMPO Azide to generate a spin label rigidly attached to one of the oligonucleotide bases, as shown in Figure 2. We anticipate that TEMPO-TEG Azide (2), with its long flexible linker, will be more suited to interrogating the general environment of the biomolecule.
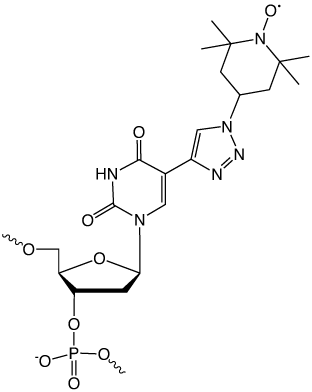
Figure 2: TEMPO-5-Ethynyl-dU Conjugate
We have used these spin labels with 5-ethynyl-dU, our Copper-Free DBCO click labels, our Oligo Click Kits, and using copper sulfate mediated Click Chemistry.
In one experiment, we prepared a mixed base 20mer oligonucleotide with a 5'-alkyne group and conjugated with TEMPO Azide using the copper sulfate procedure detailed on the following page. The resulting RP HPLC traces are shown in Figure 3. The conjugation using this procedure was virtually quantitative in four hours at room temperature.

In a second experiment, a mixed base 20mer oligonucleotide, modified at the 5' terminus with DBCO-TEG, was prepared. This oligonucleotide was conjugated with TEMPO-TEG Azide using the procedure detailed on the following page. The resulting RP HPLC traces are shown in Figure 4. Again the conjugation was essentially quantitative in one hour at room temperature.

In a third experiment to illustrate the ability of the 5-ethynyl group to click efficiently, a mixed base 20mer oligonucleotide containing a 5-Ethynyl-dU residue was conjugated with 6-FAM Azide using the copper sulfate procedure detailed on the following page. The resulting RP HPLC traces are shown in Figure 5. The conjugation efficiency using this procedure was virtually quantitative in two hours at room temperature.

5-Ethynyl-dU-CE Phosphoramidite is used in oligo synthesis with normal coupling and synthesis cycles. However, 5-Ethynyl-dU is very susceptible to base catalyzed hydration during deprotection and we have found two options for successful use as follows:
Oligos containing 5-Ethynyl-dU can be deprotected using ammonium hydroxide at room temperature. So regular protected monomers can be used with oligos containing iBu-dG being deprotected in 24 hours and those containing dmf-dG overnight.
Alternatively, UltraMild conditions with deprotection using ammonium hydroxide at room temperature for 2 hours or anhydrous 0.05M potassium carbonate in methanol for 4 hours at room temperature can be used.
Note that AMA even at room temperature and ammonium hydroxide at elevated temperatures cause significant degradation of the 5-ethynyl group.
We hope that the addition of these new spin labels, along with 5-Ethynyl-dU, will prove valuable to the growing field of EPR spectroscopy.
Click Chemistry using the Copper Sulfate Procedure
Prepare the following solutions:
- DMSO-tBuOH (3:1)
- 0.1M Copper Sulfate in water
- 0.2M TBTA in DMSO/tBuOH
- 0.1M Sodium Ascorbate (prepare fresh)
- 20mM Azide in DMSO/tBuOH
- Oligo Solution 50nmoles in 50µL
- 5'-XTT ATT CTT GTT ATT CTT GTT – 3'
- 10-1908, 5'-hexynyl phosphoramidite, 100µmol.
We prepared these solutions in 2mL HPLC vials with septum caps. The vials with loosened caps were sparged for 5-10 minutes using a fine needle to slowly bubble argon into the solutions.
Copper Catalyzed Click Chemistry, Copper Sulfate method
- Mix the TBTA/Copper Sulfate (1:1), 100µL each, degas.
- To the vial containing the oligo solution (50µL), add 50 equivalents Azide (125µL).
- Add 25 equivalents of the Copper sulfate/TBTA solution (25µL).
- Vortex. Add additional DMSO/tBuOH if a precipitate forms.
- Using a syringe with a fine needle, add 40 equivalents of 0.1M Sodium Ascorbate (20µL).
- Degas solution briefly and agitate at room temperature for 2-4 hours.
Ethanol precipitation
- Transfer to 1.5mL centrifuge tube.
- Add 20µL 3M Sodium Acetate and vortex.
- Add 520µL cold ethanol, or the amount to reach 70% ethanol, and vortex briefly.
- Chill for 30 minutes (-20°C to -70°C).
- Centrifuge for 10 minutes at 13,500rpm.
- Decant, wash twice with cold ethanol and dry.
- For downstream processes that may be sensitive to trace amounts of copper, we recommend a Glen GelPak desalting, HPLC or other suitable method.
DBCO Copper-Free Click Chemistry
- TEMPO-TEG - 5'-DBCO labelled oligo
- Dissolve 100nmole of DBCO labelled oligo in PBS, pH 7.4.
- Add 4µL of 100mM TEMPO-TEG Azide in DMSO.
- React for 1 hour at room temperature.
- Add 60µL of 3M Sodium Acetate and mix well.
- Add 1mL cold ethanol and mix well.
- Chill for 30 minutes at (-20°C to 70°C).
- Centrifuge for 10 minutes at 13,500 rpm.
- Decant, wash twice with cold ethanol and dry.
References:
- S.A. Shelke, and S. Sigurdsson, European Journal of Organic Chemistry, 2012, 2291-2301.
- I.D. Sahu, R.M. McCarrick, and G.A. Lorigan, Biochemistry, 2013, 52, 5967-5984.
- C.H. Wunderlich, et al., ACS Chem Biol, 2013, In Press.
Ordering Information
TEMPO Azide (50-2007)
TEMPO-TEG Azide (50-2008)
5-Ethynyl-dU-CE Phosphoramidite (10-1554)
- Glen Report 25.21: Methylene Blue Phosphoramidite for DNA Labelling
- Glen Report 25.22: New Product - Methylene Blue C3 Phosphoramidite
- Glen Report 25.23: New Product - 5'-Dichloro-Dimethoxy-Fluorescein Phosphoramidite
- Glen Report 25.24: New Products - TEMPO Spin Labels for Click Chemistry and 5-Ethynyl-dU
- Glen Report 25.25: New Product - 5-Formyl-dC III and the Synthesis of Oligonucleotides Containing All Four Epigenetic Nucleosides
- Glen Report 25.26: Cyanine Dyes - A Personal Perspective
- Glen Report 25.27: Technical Brief - Selective Covalent Capture of DNA and RNA Targets with Shielded Covalent Probes Incorporating a Photo-Activated Crosslinker
- Glen Report 25.28: Technical Brief - Glen UnySupport Now Available With Fast Cleavage

