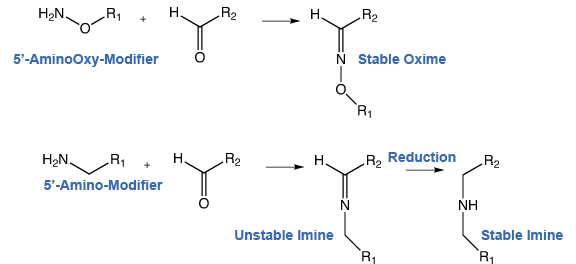
The most common conjugation reaction type by far is the reaction between an amino-modified oligonucleotide and an activated carboxylic acid to form a stable amide linkage. This relatively fast and efficient conjugation reaction is carried out at around pH9 where the exocyclic amines of the bases are inactive. However, the reaction between an amine and an aldehyde or ketone is much less useful since the imine that is formed is not stable under acidic or basic conditions and has to be reduced to a secondary amine using a borohydride reagent, such as sodium cyanoborohydride. Aminooxy modifiers may offer an alternative conjugation reaction with aldehydes and ketones since they form stable oximes.
The use of nucleophilic aminooxy modifiers for DNA conjugations was first described in the mid 1990’s.1,2 A number of improvements in oxime conjugation chemistry has led to a more reliable and direct approach for the use of aminooxy modifiers in conjugations with DNA.3,4 The oxime formed from the reaction of alkyloxyamines with aldehydes creates a stable covalent bond. In comparison, the imine formed by the conjugation of primary amines with aldehydes is not stable to acidic or basic conditions and requires subsequent reduction with borohydrides to form stable amine conjugates, as shown in Scheme 1.
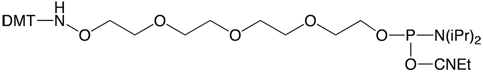
Glen Research is pleased to offer 5’-AminoOxy-Modifier 11 (1), based on a tetraethylene glycol linkage for improved solubility and for reducing the potential negative impact on hybridization of the oligo. For synthesis, we recommend using 1H-Tetrazole with a 3 minute coupling time.
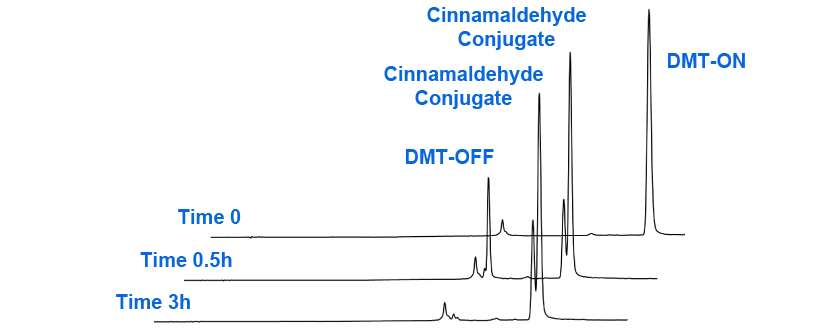
The cleanest approach we have found for solution phase conjugation is to synthesize the oligo DMT-ON and complete the deprotection with ammonium hydroxide or AMA. After drying, the conjugation can be completed in 80% acetic acid to simultaneously remove the DMT group and catalyze the oxime formation. Subsequent gel-filtration using a Glen Gel-Pak removes the acetic acid and unreacted aldehydes. This progression of oxime formation with cinnamaldehyde is illustrated in Figure 2.
The aminooxy conjugation can also be performed on the column after removal of the 5’-DMT group, provided the label is stable to the subsequent deprotection conditions.4