

Customer feedback is one of the key resources that Glen Research uses for continuous improvement of our products. Recently, a customer informed us that he was very disappointed with the performance of our 2-Amino-dA-CE Phosphoramidite (1) when added multiple times in an oligo. When oligos were synthesized containing up to four 2-amino-dA additions, the results were just acceptable but six additions proved to have a very low yield of full length oligo. Unfortunately, we were able to confirm our customer’s findings even using the optimized conditions we specify for 2-amino-dA. As shown in Figure 2, six additions of 2-Amino-dA in a mixed base 16 mer yielded a product amounting to only 13% of the oligo mixture by area of the oligo mixture when analyzed by RP HPLC. The question was then – why? - when the monomer exhibits high purity and one to three additions were achieved in high yield.
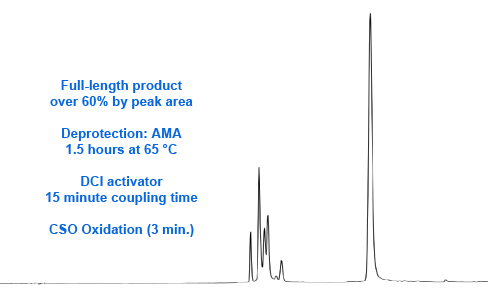
In our development work with 2-amino-dA, we investigated various activators and deblocking mixes but, at that time, we had no good alternative to iodine-containing oxidizers. Now we offer an alternative oxidizer, (1S)-(+)-(10-camphorsulfonyl)-oxaziridine (CSO) (2). 0.5M CSO in acetonitrile is a stable solution which requires a 3 minute oxidation time for regular oligonucleotide synthesis. We decided to try substituting CSO for iodine oxidation. We duly found that the synthesis of oligonucleotides with 6 additions of 2-amino-dA was enormously improved using CSO, with the percentage of full length product rising to over 60% (Figure 3).
2-Amino-dA is very susceptible to depurination if electron withdrawing protecting groups, for example acyl protection groups, are used. To stabilize the glycosidic bond, strongly electron donating formamidine protecting groups are used in this monomer. We surmise that the N2 formamidine group can activate the N3, which attacks the 3' carbon to displace the phosphonium iodide intermediate, leading to chain scission.
In situations like this where a change is required in our user instructions, we update the instructions on the analytical information and also update the Product File on our web site, in this case: 2-Amino-dA-CE Phosphoramidite (10-1085).

2-Amino-dA-CE Phosphoramidite (10-1085)
0.5M CSO in Anhydrous Acetonitrile (40-4632)