Chemical synthesis of RNA is still in an early stage of development: monomers are expensive to produce; coupling yields per cycle are substantially lower than in DNA; effective synthesis scales remain quite small; and post-synthetic deprotection steps are quite involved and time consuming. Consequently, a large percentage of RNA synthesis is carried out enzymatically using the method known as runoff transcription.(1) However, problems also exist in this enzymatic technique. In copying the DNA template, the polymerase enzyme sometimes fails to stop at the last coding base and the desired RNA sequence is contaminated with sequences containing one to several additional bases. This situation gives rise to a purification challenge since the contaminating RNA sequences are similar in length. Also, if the target RNA is to be isotopically labelled, the purification results in loss of expensive label.
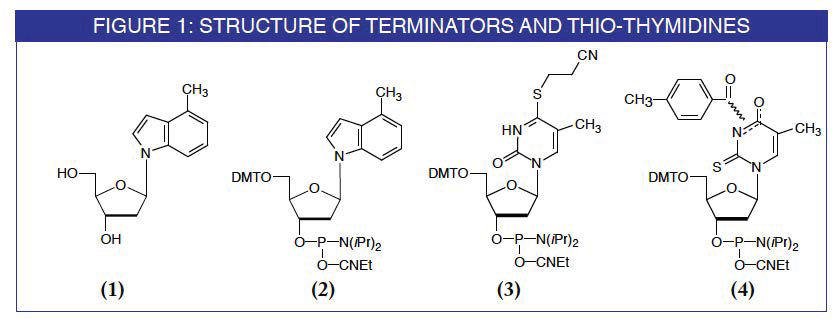 The need to terminate RNA synthesis by somehow interrupting the polymerase led to the evaluation of several non-polar, non-hybridizing nucleoside analogues by a group at the University of Rochester.(2 ) When the indole derivative was placed at the 5'-terminus of DNA templates, a significant improvement in quality of the transcribed RNA resulted. Without termination, longer undesired RNA sequences amounted to about 45% of the desired product RNA. This was reduced by more than 2-fold to below 20% when the template had the indole base analogue at the 5' terminus. The indole derivative has also been shown to be even more efficient at terminating enzymatic DNA synthesis, cutting the level of n+1mer by approximately 10-fold.(2)
The need to terminate RNA synthesis by somehow interrupting the polymerase led to the evaluation of several non-polar, non-hybridizing nucleoside analogues by a group at the University of Rochester.(2 ) When the indole derivative was placed at the 5'-terminus of DNA templates, a significant improvement in quality of the transcribed RNA resulted. Without termination, longer undesired RNA sequences amounted to about 45% of the desired product RNA. This was reduced by more than 2-fold to below 20% when the template had the indole base analogue at the 5' terminus. The indole derivative has also been shown to be even more efficient at terminating enzymatic DNA synthesis, cutting the level of n+1mer by approximately 10-fold.(2)
The phosphoramidite of the 4-methylindole derivative is now available from Glen Research, under license from the University of Rochester.
Incorporation of modified bases into synthetic oligonucleotides offers researchers the ability to study unnatural DNA functionality. Experiments may be designed to evaluate the effect of a given modification on DNA DNA, DNA-RNA or DNA-protein interaction. In this way, researchers can examine hybridization or conformation of nucleic acids or elucidate the mechanism of, say, nuclease or polymerase interactions. One versatile and simple modification is the replacement of oxygen with sulfur on nucleobases. We now offer two thio derivatives of thymidine for investigation of oligonucleotide structure and activity.
4-Thio-dT is a very useful modification which can be used for photo crosslinking and photoaffinity labelling experiments.(3) The thiocarbonyl group is also amenable to post-synthetic modification.(4) Although oligos containing 4-thio-dT can be accessed using our convertible dT monomer, routine preparation is significantly simplified using the protected monomer. Although synthesis with this monomer proceeds conventionally, we recommend a modification of the deprotection step to preserve the thiocarbonyl group. To the standard ammonium hydroxide solution, sodium hydrosulfide (NaSH) is added to a concentration of 50mM. This minimizes ammonolysis of the S-cyanoethyl group.
Similarly, oligos containing 2-thio-dT are useful in examining protein DNA interaction by acting as photolabile probes. The thiocarbonyl group in 2-thio-dT is especially interesting in that it is available to react with compounds associating with the minor groove of DNA. A monomer protecting scheme which prevents desulfurization and degradation of 2-thio-dT by oxidation during oligonucleotide synthesis has been described.(5 ) The rather strange looking monomer uses a toluyl protecting group which is located at the N3 or O4 position. This group is removed quantitatively during standard deprotection with ammonium hydroxide.
(1) J.F. Milligan, D.R. Groebe, G.W. Witherell, and O.C. Uhlenbeck, Nucleic Acids Res., 1987, 15, 8783-8798.
(2) S. Moran, X.-F. Ren, C.J. Sheils, S. Rumney IV, and E.T. Kool, Nucleic Acids Res., manuscript submitted.
(3) T.T. Nikiforov and B.A. Connolly, Nucleic Acids Res., 1992, 20, 1209 1214.
(4) R.S. Coleman and E.A. Kesicki, J. Amer. Chem. Soc., 1994, 116, 11636-11642.
(5) R.G. Kuimelis and K.P. Nambiar, Nucleic Acids Res., 1994, 22, 1429 1436.
4-Methylindole-CE Phosphoramidite: (10-1045) has been discontinued.
4-Thio-dT-CE Phosphoramidite (10-1034)
2-Thio-dT-CE Phosphoramidite (10-1036)