Glen Report 8.23: Photolabile Support for the Preparation of 3'-Carboxylates
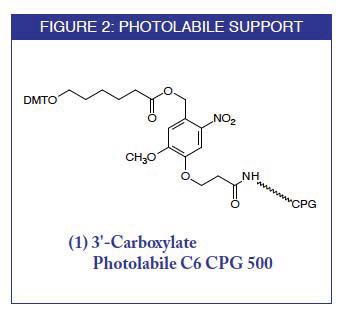
A variety of techniques exist for preparing oligonucleotides modified at the 5'-terminus or within the sequence but 3'-modification remains limited to amino and thiol (sulfhydryl) groups. Moreover, the vast majority of currently available commercial supports lead to the removal of at least a portion of the nucleobase and phosphate protecting groups during cleavage of the oligonucleotide from the support. (The exception is the ribonucleoside supports for DNA modification(1,2) and oxidizable solid support(3) which yield a fully base-protected oligonucleotide 3'-phosphate after oxidative cleavage and ß-elimination.) We are now introducing a universal, photolabile support for the preparation of an oligonucleotide 3'-carboxylate with or without the base protecting groups. The development of a commercially viable photolabile support extends from research carried out by Marc Greenberg and his group at Colorado State University.(4)
 The research goals which culminated in the development of the support are detailed below:
The research goals which culminated in the development of the support are detailed below:
- Oligonucleotides containing 3'-terminal carboxylic acids should be isolated in normal yield by ammonium hydroxide deprotection.
- Photolytic cleavage from the support should result in the release of a fully protected oligonucleotide 3'-carboxylic acid.
- Photolytic cleavage from the support should occur under conditions which lead to minimal damage of the product oligonucleotide.
- The support should be independent of the base at the 3'-terminus, i.e., a universal support, the first 3'-base being added during the first phosphoramidite coupling cycle.
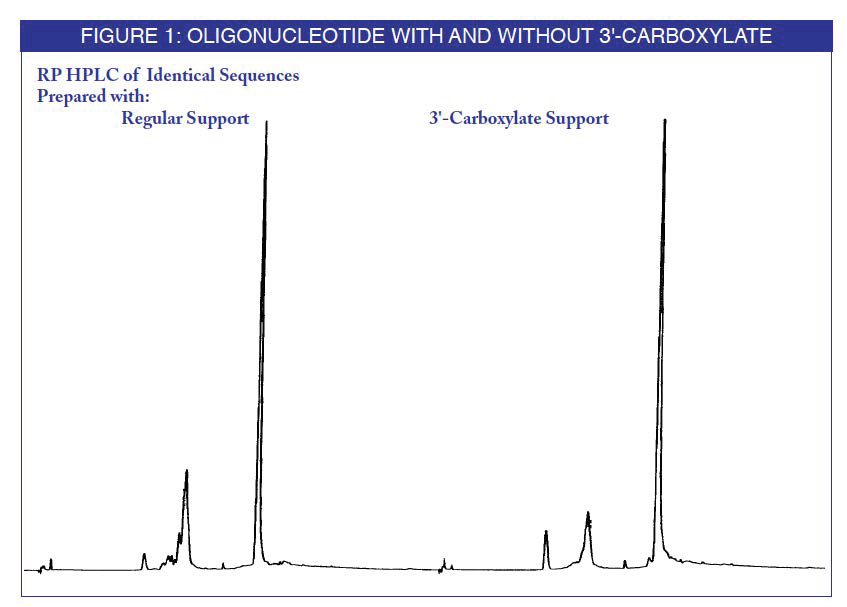
An oligonucleotide synthesized on the support was compared to the equivalent oligonucleotide with no 3'-substitution. After ammonium hydroxide cleavage and deprotection, the products were analyzed by reverse phase (RP) HPLC. The mobility change when using the photolabile support is indicative of the formation of the desired 3'-carboxylate
To compare photochemical cleavage with ammonium hydroxide cleavage, the same oligonucleotide was cleaved by photolysis from the support. The oligonucleotide was then deprotected with ammonium hydroxide and analyzed. The 3'-carboxylate products were found to be identical.(4)
The conditions chosen for irradiation of the product oligo-nucleotides have been shown to cause less than 1% thymidine dimer formation, as a measure of photoinduced damage. The yields of the oligonucleotides isolated by photo cleavage are reported to be about 30% less than those from ammonium hydroxide cleavage. To date, our experiments have been carried out using a TLC transilluminator (long wavelength UV) rather than a Hg/Xe lamp at 400nm
Glen Research is happy to offer this photolabile carboxylate C6 support under license from Colorado State University.
References
(1) M. Lemaitre, C. Bisbal, B. Bayard, and B. Lebleu, Nucleosides & Nucleotides, 1987, 6, 311-315.
(2) M. Lemaitre, B. Bayard, and B. Lebleu, Proc. Natl. Acad. Sci. USA, 1987, 84, 648-652.
(3) R. Lohrmann, L. Arnold, and J.L. Ruth, DNA, 1984, 3, 122.
(4) D.J. Yoo and M.M. Greenberg, J. Org. Chem., 1995, 60, 3358-3364.
Product Information
3'-Carboxylate Photolabile C6 CPG 500: Discontinued
- Glen Report 8.21: 5'-Labelling - Fluorescein and Cyanine Dyes, Biotin
- Glen Report 8.22: 5'-Phosphorylations - Now Compatible with DMT-On Purification
- Glen Report 8.23: Photolabile Support for the Preparation of 3'-Carboxylates
- Glen Report 8.24: Transcription Terminator, 2-Thio- and 4-Thio-Thymidine
- Glen Report 8.25: Rambo Activator - Ethylthiotetrazole

