Xianbin Yang
AM Biotechnologies
6023 Ave S.
Galveston, Texas 77551
Replacing two non-bridging oxygen atoms with sulfur atoms in a DNA phosphodiester linkage creates a phosphorodithioate (PS2) linkage.1 Like natural DNA, the phosphorodithioate linkage is achiral at phosphorus. Additionally, recent research has demonstrated that this analog is completely resistant to nuclease degradation and forms complexes with DNA and RNA with somewhat reduced stabilities.2 Moreover, it has been found that PS2-ODNs bind proteins with a higher affinity than their phosphodiester analogues2-6 suggesting that PS2-ODNs may have additional utility in the form of sulfur-modified phosphate ester aptamers (thioaptamers)3,6-8 for therapeutic and diagnostic applications. The biological interest and promise of the PS2-ODNs has spawned a variety of strategies for synthesizing, isolating, characterizing and purifying this compound over the past two decades.9 However, the thiophosphoramidite building blocks necessary to synthesize a PS2-ODN have only now become commercially available after recent work at AM Biotechnologies (www.thioaptamer.com). This article highlights a working protocol that enables the widespread use of thiophosphoramidites for the synthesis of PS2-ODNs.



Synthesis columns, thiophosphoramidites, and synthesis reagents were obtained from Glen Research, Sterling, VA. Dichloromethane (DCM) anhydrous (#270997), 3Å Molecular Sieve beads (8-12 mesh, #208582), DL-dithiothreitol (#D9779-1G), Ammonium hydroxide (#338818), HPLC grade ethanol (#459828) were from Sigma-Aldrich.
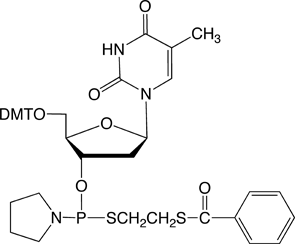
The structures of thiophosphoramidites (thioPA) are shown in Figure 1. Like normal DNA and RNA phosphoramidites, the dried solid form of the thioPAs is very stable at -20°C for at least one year based on 31P-NMR analysis with no observed reduction in reactivity for synthesizing the PS2 linkage. However, a few simple modifications to standard synthesis protocols are necessary when using the thioPAs.
A typical cycle for the solid-phase synthesis of a PS2 linkage is different from a standard cycle for the synthesis of normal phosphate linkages. For the PS2 linkage synthesis, following 3% TCA detritylation of a 2’-deoxynucleoside linked to the solid support, the thioPA is coupled to the free 5’ hydroxyl group on the support using an activator such as DCI or tetrazole. The resulting thiophosphite triester is then sulfurized by either 3-ethoxy-1,2,4-dithiazolidine-5-one (EDITH) or 3-((N,N-dimethyl-aminomethylidene)amino)-3H-1,2,4-dithiazole-5-thione (DDTT) reagent. Unreacted support-linked 2’-deoxynucleoside is then capped, which completes the cycle for addition of the nucleotide.


Tetrazole has been commonly used as an activator of choice for phosphoramidite chemistry. However, unpublished studies have shown that tetrazole is not the most efficient activator for the thioPA coupling reaction. It is imperative to use an efficient activator during commercial PS2 production.
Activators that are commercially available include 5-ethylthio-1H-tetrazole (ETT), 5-benzylthio-1H-tetrazole (BTT), 4,5-dicyanoimidazole (DCI), and 5-(bis-3.5-trifluoromethylphenyl)-1H-tetrazole (Activator-42). The coupling efficiency of all of these activators during PS2 synthesis was compared on an Expedite 8909 DNA synthesizer. Significant differences were observed, which suggests that the thioPA activation mechanism differs from that of the normal phosphoramidite. Therefore, a practical protocol for use on an Expedite 8909 DNA synthesizer was developed for the commonly used tetrazole activator; however, another protocol for the DCI activator, which is more efficient for PS2 linkage synthesis, was also developed.
(https://www.glenresearch.com/media/productattach/import/tbn/Expedite_8909_PS2_Tetrazole_Protocol.pdf)
A dimer model sequence, 5’-XPS2T-3’, was used to study the thioPA coupling efficiency. Tetrazole was used as the activator and DDTT as a sulfurizing reagent. The experiments demonstrated that to achieve > 93% coupling yield for all of the thioPAs, a coupling time of 9 minutes was required. Even the dC-thioamidite exhibited a very slow rate (Figure 2) of PS2 linkage formation using tetrazole.
(https://www.glenresearch.com/media/productattach//e/x/expedite_8909_ps2_dci_protocol.pdf)
DCI is emerging as an alternative activator to tetrazole. DCI is soluble in acetonitrile up to 1.1 M at room temperature and experiments with thioPAs have shown that DCI decreases the time required to achieve up to 96% coupling yield by a factor of three as compared with tetrazole (Figure 2).
Beaucage Reagent is a very popular sulfurizing agent for the synthesis of phosphoromonothioate linkages using normal phosphoramidites. When Beaucage Reagent was used with the thioPAs to synthesize PS2 linkages, it was observed that the by-product formed in the sulfurization reaction oxidizes the thiophosphite triester. This oxidation leads to phosphoromonothioate by-products, thus lowering the desired product yield and complicating the purification of the desired product. In addition, Beaucage Reagent has a tendency to precipitate from solution and clog the solvent and reagent transfer lines of a DNA synthesizer. DDTT is another sulfurizing agent primarily used to synthesize RNA phosphoromonothioates; however, it can be used to synthesize DNA phosphoromonothioates as well. Comparing DDTT efficiency to Beaucage Reagent in sulfurizing PS2 linkages, it was found that DDTT has slightly better sulfurizing reactivity than Beaucage Reagent (Figure 3); however, importantly, DDTT reduces the formation of phosphoromonothioate linkages during the synthesis of PS2 linkages thus increasing product yield.
Upon completion of the automated synthesis, the support was removed from the synthesizer and dried with argon. The support was transferred into a 4 mL sealable vial where 1 mL of concentrated ammonia:ethanol (3:1, V:V) mix containing 20 mM DTT was added to the vial. The vial was sealed and incubated at 55 °C for 15-16 h. After the vial was removed from the oven and cooled to room temperature, the solution was transferred to a larger vial and 4~5 mL of distilled water was added. Solvents were removed by lyophilization.
The above protocol was used to synthesize a model sequence: 5’-TPS2TPS2TPS2TPS2TPS2T-3’. The 31P-NMR spectra are provided in Figure 4.

It should be noted that the Expedite 8909 oxidizing time as well as the oxidizing reagent volumes found in the manufacturer’s protocol are not sufficient to synthesize chimeric DNA containing PS2 linkage(s). The protocols referenced above correct these problems.
Glen Research has also developed a PS2 synthesis cycle for the ABI394 DNA synthesizer using regular tetrazole or 0.25M DCI activators.
(https://www.glenresearch.com/media/productattach/import/tbn/ABI_394_PS2_Protocol.pdf)