Glen Report 20.18: New Products - Solid CPR II / Formylindole – Aldehyde Modifier
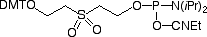
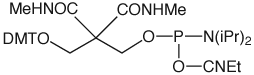
CPR II (1) has become1 a very popular chemical phosphorylation reagent (CPR) for phosphorylating oligos at the 5’ terminus. While CPR II is most commonly used DMT-on to allow simple cartridge purification of the oligos produced, it can also be used DMT-off if the 5’-phosphorylated oligos can be used without purification in the same way as our original CPR (2).2 One minor drawback in the usage of these two CPRs is the fact that they are both viscous oils. We offer these products prepackaged in septum-capped vials but it is sometimes useful in high throughput situations to be able to weigh powder into a bottle in the exact quantity needed for the synthesis session. The answer is simple – Solid CPR II (3).
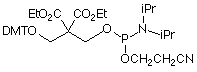
Reagent II
Reagent
Reagent II
Solid CPR II is the dimethylamide analogue of CPR II so it is more stable than CPR II to the conditions of oligonucleotide synthesis. Consequently, it can be used at the 5’ terminus without the need to remove the capping step in the last cycle, which is the case for CPR II. It can also be used at the 3’ terminus in situations where CPR is too labile for the synthesis cycles and any special manipulations during the synthesis. For example, this amide structure was used3 for the synthesis of a long oligo in which a silyl protecting group had to be removed with a fluoride reagent, which proved to be too basic for our standard 3’-phosphate support.
If the final DMT is removed during synthesis, deprotection under standard conditions will eliminate the Solid CPR II to yield a 5' Phosphate. If the DMT is retained, see technical bulletin for removal procedure.
References
- A. Guzaev, H. Salo, A. Azhayev, and H. Lonnberg, Tetrahedron, 1995, 51, 9375-9384.
- T. Horn, and M. Urdea, Tetrahedron Lett., 1986, 27, 4705.
- P.J. Brooks, et al., J Biol Chem, 2000, 275, 22355-62.
Product Information
Formylindole – Aldehyde Modifier
Conjugation of biopolymers using aldehyde intermediates is becoming increasingly popular1,2 since the reaction of an aldehyde with oximes, hydrazines and semicarbazides is fast and highly specific. In 2003, Glen Research launched our first aldehyde modifier, 5’-aldehyde modifier C2 (4).3 This patented product is effective and easy to use and is offered by Glen Research under a license agreement with Epoch/Nanogen. While this product may be used for all research and development purposes, IVD developers would usually prefer to use a product with no intellectual property (IP) issues. At the time of our review of aldehyde modifiers, we also evaluated the formylindole phosphoramidite (5), which had just been described4 by researchers at Kyoto University. As the interest in aldehyde modifiers grows, we have concluded that this product has a place in our catalog and we are happy to make it available to our customers.
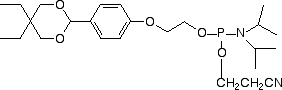
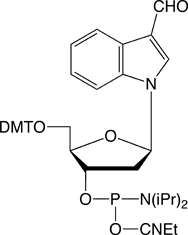
This product is simplicity personified and there is no need to change any of the regular synthesis, cleavage and deprotection conditions when using it. The aldehyde is stable during deprotection and the DMT group is available for DMT-on purification, if desired. The aldehyde is still sufficiently active to conjugate extremely well with reagents containing oximes, hydrazines and semicarbazides.
References
- T.S. Zatsepin, D.A. Stetsenko, M.J. Gait, and T.S. Oretskaya, Bioconjugate Chemistry, 2005, 16, 471-489.
- T.S. Zatsepin, D.A. Stetsenko, M.J. Gait, and T.S. Oretskaya, Tetrahedron Lett, 2005, 46, 3191-3195.
- M.A. Podyminogin, E.A. Lukhtanov, and M.W. Reed, Nucleic Acids Res, 2001, 29, 5090-8.
- A. Okamoto, K. Tainaka, and I. Saito, Tetrahedron Lett, 2002, 43, 4581-4583.
Product Information
5'-Aldehyde-Modifier C2 Phosphoramidite (10-1933)
5-Formylindole-CE Phosphoramidite (10-1934)
- Glen Report 20.11: An Unnatural Base Pair System for the Expansion of Genetic Information
- Glen Report 20.12: Thiophosphoramidites and Their Use in Synthesizing Oligonucleotide Phosphorodithioate Linkages
- Glen Report 20.13: Technical Brief - Purification of 6-FAM Labelled oligos using Glen-Pak™ Cartridges
- Glen Report 20.14: More Click Chemistry
- Glen Report 20.15: Technical Brief - New application of 5-Me-iso-dC and iso-dG
- Glen Report 20.16: Technical Brief - New application for 5’-OMe-dT phosphoramidite
- Glen Report 20.17: New Product - Deuterated 2’-Deoxyguanosine Phosphoramidite
- Glen Report 20.18: New Products - Solid CPR II / Formylindole – Aldehyde Modifier
- Glen Report 20.19:New Product - 5'-Cholesteryl-TEG Phosphoramidite
- Glen Report 20.110: Improving Universal Support II for Oligonucleotide Synthesis
- Glen Report 20.111: Differences Between Universal Support II and III
- Glen Report 20.112: Technical Brief - Phage Display, Artificial Antibodies and Trimer Phosphoramidites

