Glen Report 20.19:New Product - 5'-Cholesteryl-TEG Phosphoramidite
Cholesteryl labelling of oligos continues to find favor, especially in the field of therapeutics: antisense1-3 and siRNA4-7. Since oligonucleotides are predominantly hydrophilic, they tend to have difficulty permeating cell membranes. In order to improve cellular uptake, one strategy is to conjugate to oligonucleotides molecules that are non-toxic and hydrophobic, such as cholesterol. And it is relatively simple to modify oligonucleotides at the 3' or 5' terminus with cholesteryl-TEG.
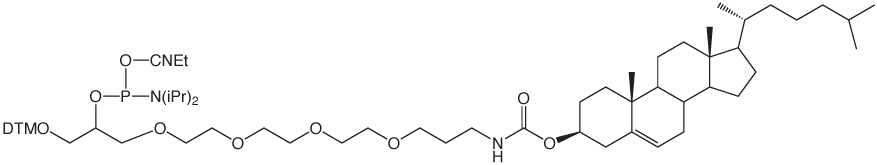
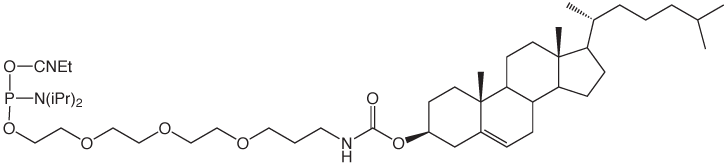
Several of our customers have asked for a change in the structure of our existing product, Cholesteryl-TEG phosphoramidite (1). This product uses the popular tetraethylene glycol (TEG) branched spacer originally introduced many years ago, which allows multiple insertions of the attached tag. The problem is that the cholesterol molecule is so hydrophobic that one addition per oligo terminus is more than enough. Consequently, Cholesteryl-TEG Phosphoramidite is normally added only once at the 5’ terminus and there is no need for its capacity for multiple additions. The DMT group and the underlying 1,2-diol then become a liability rather than an asset. A minor structural adjustment leads us to 5’-Cholesteryl-TEG Phosphoramidite (2).
5'-Cholesteryl-TEG Phosphoramidite is dissolved in acetonitrile, which is in contrast with Cholesteryl-TEG Phosphoramidite which requires 10% THF in acetonitrile for solubility. A coupling time of 3 minutes with tetrazole as activator is optimal for 5'-Cholesteryl-TEG Phosphoramidite. Cholesterol is VERY hydrophobic so oligos prepared using 5'-Cholesteryl-TEG Phosphoramidite are easily purified by reverse phase techniques, including reverse phase cartridges.
References
- M.K. Bijsterbosch, et al., Nucleic Acids Res., 2000, 28, 2717-2725.
- M.K. Bijsterbosch, et al., J. Pharmacol. Exp. Ther., 2002, 302, 619-626.
- M. Manoharan, Antisense Nucleic Acid Drug Dev, 2002, 12, 103-28.
- M. Manoharan, Curr Opin Chem Biol, 2004, 8, 570-9.
- J. Krutzfeldt, et al., Nature, 2005, 438, 685-9.
- J. Krutzfeldt, et al., Nucleic Acids Res., 2007, 35, 2885-2892.
- C. Wolfrum, et al., Nat Biotechnol, 2007, 25, 1149-57.
Product Information
- Glen Report 20.11: An Unnatural Base Pair System for the Expansion of Genetic Information
- Glen Report 20.12: Thiophosphoramidites and Their Use in Synthesizing Oligonucleotide Phosphorodithioate Linkages
- Glen Report 20.13: Technical Brief - Purification of 6-FAM Labelled oligos using Glen-Pak™ Cartridges
- Glen Report 20.14: More Click Chemistry
- Glen Report 20.15: Technical Brief - New application of 5-Me-iso-dC and iso-dG
- Glen Report 20.16: Technical Brief - New application for 5’-OMe-dT phosphoramidite
- Glen Report 20.17: New Product - Deuterated 2’-Deoxyguanosine Phosphoramidite
- Glen Report 20.18: New Products - Solid CPR II / Formylindole – Aldehyde Modifier
- Glen Report 20.19:New Product - 5'-Cholesteryl-TEG Phosphoramidite
- Glen Report 20.110: Improving Universal Support II for Oligonucleotide Synthesis
- Glen Report 20.111: Differences Between Universal Support II and III
- Glen Report 20.112: Technical Brief - Phage Display, Artificial Antibodies and Trimer Phosphoramidites

