
The purine 6-thioguanine1 has been used for many years in the treatment of human malignancies, especially leukemias. It is likely that purine is converted into the nucleoside 6-thioguanosine in DNA and RNA and the cytotoxic effects may be due to damage caused by strand cleavage or cross-linking to DNA and proteins. The physico-chemical basis for these biological effects is the reactive thiocarbonyl group and oligonucleotide researchers have exploited this reactivity in several ways.
The interaction of DNA with DNA binding proteins can be studied using photochemically induced cross-linking. Predominantly, studies have been carried out using 5-bromo- and 5-iodo-pyrimidines but 4-thiopyrimidines have also been used. With the advent of phosphoramidites of thionucleosides, photoaffinity labelling experiments using these modified bases are now readily possible. For example, 4-thiothymidine and 6-thiodeoxyguanosine (S6-dG) were shown2 to cross-link effectively with Eco RV endonuclease and methyltransferase. The advantages of using the thio derivatives for photo cross-linking include their similarity to the natural structures, and the wavelength required, 340 nm, which is removed from the maxima of the regular bases and should cause no other damage. In addition to its photochemical reactivity, nucleobases containing thiocarbonyl groups can also be chemically modified selectively at the sulfur position by alkylating reagents.3
6-Thiodeoxyguanosine has also been incorporated into G-rich triple helix-forming oligonucleotides. Replacement of all or some G residues in G-rich oligonucleotides with S6-dG has been shown4 to inhibit self association and formation of G tetrads, especially in potassium buffers. This allows triple helix formation to take place normally.
Our new S6-dG monomer (1, Figure 1) has the S6 position protected with cyanoethyl and N2 with trifluoroacetyl protecting groups. After normal synthesis, the synthesis column with oligonucleotides containing S6-dG should be treated5 with 1M 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in anhydrous acetonitrile at room temperature for 5 hours to remove the S6-cyanoethyl protecting group. The oligo deprotection is completed with 50 mM sodium hydrosulfide (NaSH) in ammonium hydroxide at room temperature for 24 hours.
RNA modification is currently in vogue for such applications as antisense and ribozymes. Interesting changes in RNA activity can be effected by substituting the 2'-hydroxyl with 2'-fluoro or 2'-O-alkyl groups. A further obvious substitution would be the 2'-amino group.
The thermal stability of duplexes containing 2'-amino-RNA has been determined6 and it was reported that 2'-amino-C substitutions destabilized by about 4° relative to RNA C. It was also further reported that 2'-amino-RNA linkages are nuclease-resistant.
The pKa of the 2'-amino group is quite low at 6.2 but this retains sufficient nucleophilicity to allow conjugation reactions to take place. It is therefore possible to label a 2'-amino group with a fluorophore like rhodamine. This activity has been used rather elegantly to investigate7 thermal motion in a large ribozyme. The 2'-position within an RNA duplex is directed towards the outside of the helix in a location which is very amenable to interhelix contact. The researchers were able to conjugate a disulfide group to the 2'-amino group via an activated ester to yield intermediates (1) or (2), as shown in the figure below. An exchange reaction between the activated disulfide and a thiol in the complementary section or strand neatly forms a disulfide cross-link.
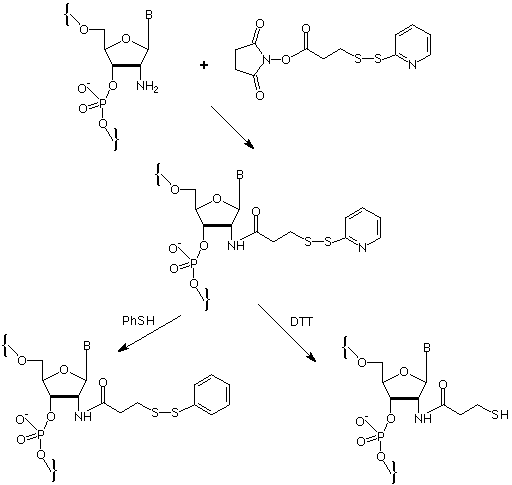
It is worthy of note that complex oligonucleotides containing 2'-amino-RNA residues benefit considerably from the use of 4,5-dicyanoimidazole (DCI)8 as activator.
We are happy to offer 2'-Amino-C (2) and 2'-Amino-U (3) monomers conveniently protected with base-labile groups which are removed by regular treatment with ammonium hydroxide.

Free-radically induced DNA damage and attendant enzymatic repair systems continue to be research topics of more than passing interest. Glen Research has made it a goal to introduce oxidatively damaged deoxynucleosides in forms suitable for automated DNA synthesis. Our latest introductions are the oxidized cytidine nucleoside 5-Hydroxy-2'-deoxyCytidine (5-OH-dC) and its deamination product 5- hydroxy-2'-deoxyUridine (5-OH-dU) as phosphoramidites. These lesions are observed in liver, kidney and brain DNA, at fairly constant levels, indicating that repair systems are not totally effective with obvious implications to the aging process. The enzymatic repair of these two lesions, as well as their role in mutagenesis, are clearly worthy of study.
The synthesis of these two monomers and their incorporation into oligos was recently described9 by a group from MIT. The reactive 5-OH group was protected in both cases with acyl protecting groups and this simple protection scheme proved to be perfectly compatible with regular synthesis and deprotection.

Sometimes we prepare products for which there is no known use and little logic for their creation. Here are two spacer products which are looking for good homes. Spacer 9 CPG is absolutely the ideal product for preparing an oligonucleotide with its 3'-terminus blocked with a triethyleneglycol group. So, if you need to block polymerase extension with a mixed polarity polyether, you need look no further. Spacer C18 Phosphoramidite promises to be the product of choice for adding a very hydrophobic section to an oligonucleotide. There, we just knew a silent minority of our customers was fervently awaiting these below-average innovations.
6-thio-dG-CE Phosphoramidite (10-1072)
5-OH-dC-CE Phosphoramidite (10-1063)
5-OH-dU-CE Phosphoramidite (10-1053)
Spacer C12 CE Phosphoramidite ((10-1928)
Spacer 9 CPG (20-2919), Spacer C18 CE Phosphoramidite (10-1928), 2'-NH2-C Phosphoramidite(10-3510) and 2'-NH2-U Phosphoramidite(10-3530) have been discontinued