Dr. Patrick Erbacher, CSO
Polyplus-transfection SA
Bioparc, 850 Boulevard Sébastien Brant
67400 Illkirch, FRANCE


ZNA® are a novel class of modified oligonucleotides enhancing hybridization properties of nucleic acids. ZNA are oligonucleotides conjugated to cationic units. Their global charge can be modulated by the number of cationic spermine moieties grafted on each oligonucleotide. ZNA are produced using standard automated nucleic acid synthesis based on phosphoramidite chemistry. A spermine derivative (i.e., spermine phosphoramidite synthon) was specifically developed as an N-protected trityl (DMT) spermine phosphoramidite that can be introduced during the oligonucleotide synthesis.1 This spermine addition chemistry is robust and versatile.2 In addition, coupling yields for such addition are high and comparable to the coupling yields obtained with the standard protected nucleobases, as described in a previous Glen Report.3
With such versatile ZNA chemistry, ZNA technology is applicable to any application involving oligonucleotides. However, the global charge of ZNA defines per se the fields of applications.
When negatively charged, ZNA conserve their ability to mediate specific hybridization with complementary strands and they are more suitable for PCR applications, amplification techniques, diagnostic applications or purification technologies.4,5
On the contrary, positively charged ZNA are more likely to mediate first a non-specific binding/interaction with polyanions through electrostatic interactions before their possible specific hybridization to a target sequence through base pairing. ZNA molecules bearing excess cationic charges were shown to have the property to stick to anionic cell surfaces and to trigger endocytosis, leading to cell transfection without the need of a transfection reagent (or a cargo).6 As a proof-of-principle, cationic ZNA-based siRNAs were shown to perform effective and specific gene silencing in animals cells without the need of a transfection reagent to mediate the cell uptake.7
Hybridization studies demonstrated that spermine-mediated attraction is the main driving force for the initiation of nucleic acid binding. Then, the standard base pairing, according to Watson-Crick rules, between the target strand sequence and the complementary ZNA leads to a duplex formation. As a consequence of the cationic contribution, the thermal stability of the formed duplex is increased. The stepwise spermine conjugation raises the Tm in a smooth and linear way while preserving mismatch discrimination. According to the initial study from Noir, et al.,8 the melting temperature (Tm) increase of ZNA brought about by spermine conjugation only depends on the length of the oligonucleotide and on the number of spermines grafted on it, providing a way to predict and finely tune the Tm of any given sequence. In this study, the impact of the number of grafted spermines per oligonucleotide on the melting temperature was determined, with 6-, 8-, 10-, 14-, or 20-mer oligonucleotides bound to complementary sequences within 45-59-mer oligonucleotides (see Figure 1).
Name |
Sequence |
GC content |
Tm |
|
Anti-miR9-22 |
TCATACAGCTAGATAACCAAAG |
36.4% |
59.5°C |
|
Anti-miR9-20 |
TCATACAGCTAGATAACCAA |
35% |
57.8°C |
|
Anti-miR9-18 |
TCATACAGCTAGATAACC |
38.9% |
54.6°C |
|
Anti-miR9-16 |
TCATACAGCTAGATAA |
31.3% |
49.2°C |
|
Anti-mir124a-22 |
TGGCATTCACCGCGTGCCTTAA |
54% |
70.7°C |
|
Anti-mir124a-20 |
GGCATTCACCGCGTGCCTTA |
60% |
69.2°C |
|
Anti-mir124a-18 |
CATTCACCGCGTGCCTTA |
55.6% |
64.5°C |
|
Anti-mir124a-16 |
ATTCACCGCGTGCCTT |
56.3% |
64°C |
|
N-22 |
ACCTGGCTCCCTCGCCTGTCAC |
68.2% |
73.1°C |
|
N-20 |
ACCTGGCTCCCTCGCCTGTC |
70% |
71.7°C |
|
N-18 |
ACCTGGCTCCCTCGCCTG |
72.2% |
70.1°C |
|
N-16 |
ACCTGGCTCCCTCGCC |
75% |
68.1°C |
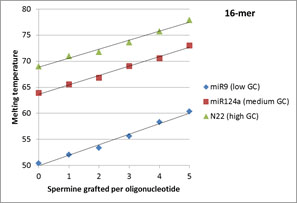
In this initial study, the melting temperatures were measured in physiological ionic strength (150 mM sodium chloride) and at pH 7.4. Additional measurements were made in order to study the impact of the ΔTm increase per spermine in more standard PCR conditions using a PCR-like buffer (Tris-HCl 10 mM pH 8.5, KCl 50 mM, MgCl2 3 mM) and using a complementary target containing 55 bases. Three groups of sequences with various GC contents (low, medium, and high) and standard lengths of primers and probes (22 to 16 mers) were synthesized (see Table 1). For each group, the length of sequences was gradually decreased, while maintaining a similar GC content for each group and the equal stability of both 3’ and 5’ sides. Each sequence was modified with an increasing number of spermines. The maximum number of grafted spermines was determined taking into consideration the solubility of the molecule (i.e., with a negative global charge). Finally, the melting temperature was measured.
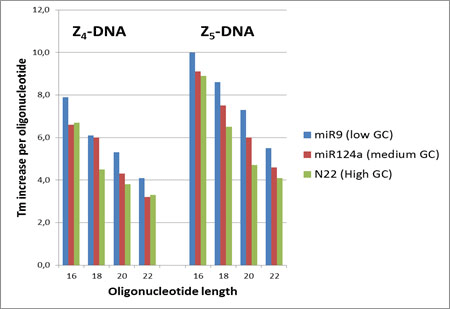
Tm measurements in PCR buffer conditions indicate that the Tm increase is linear and is a function of the number of spermines grafted to the oligonucleotide, as shown in Figure 2, which presents the results obtained with 16- and 22-mers. The linear increase per conjugated spermine was also confirmed for the 18- and 20-mers. This study also demonstrates that the Tm increase induced by the spermine grafting is lower in GC-rich oligonucleotides. It also provides additional explanation further to prior studies4 about why, when ZNA are used as primers, the maximal improvement effect on the amplification is obtained with AT-rich sequences.

Many recent reports have described the use of ZNA as potent primers and probes for PCR applications, which are mostly based on Z4- and Z5-conjugated oligonucleotides with lengths of 16 to 22 nucleotides. Figure 3 is a summary of the ΔTm increase per oligonucleotide in these selected classes of ZNA and shows that the Tm remains predictable, approximately following the previous published rules8 even in PCR buffer conditions. The Tm increase per oligonucleotide remains a function of the number of spermines grafted onto the oligonucleotide, is dependent on the oligonucleotide length, and is slightly dependent on the GC content. However, the Tm impact per spermine can be affected by the pH which depends on the buffer used and on the salt content having a shielding effect on the cationic charge. Since the composition of PCR Master Mixes is rarely provided, it is therefore recommended to find the optimal conditions and design for the ZNA. The Mg2+ concentration is critical for ZNA primers' activity (ideally 1.5 mM). However, the addition of EDTA can improve the ZNA primers' performances by depleting the excess of free Mg2+ in some PCR Master Mixes.4
Recent publications highlight the high sensitivity of ZNA when used as probes such as dual-labelled hydrolysis probes for microRNAs,9 polymorphism/mutation10 or detection of rare disease-associated transcripts11 by qPCR. A representative probe structure consists of 5’-FAM, 18/24-mer probe length, 3’-Z4/5, and 3’-BHQ-1.
The versatile chemistry of ZNA offers various possibilities of primer and probe chemical structures, such as dual-labelled probes. The intrinsic properties of ZNA drive their potency in PCR applications. Their properties lead to an increased affinity with nucleic acids due to low electrostatic repulsion, an increased hybridization and stability with the target sequence (Tm increase), a preserved capacity of single base discrimination (target specificity), and a preserved activity as substrates of polymerases.
Oligonucleotides cannot enter living cells by themselves due to their negative charge. To date, oligonucleotides are formulated into particles using cationic polymers, such as Polyplus-transfection reagent jetPEI® or lipid compounds to improve their delivery, or when conjugated to cationic peptide moieties such as cell penetrating peptides. The ZNA chemistry was used to produce cationic antisense12 or siRNA7 conjugates via automated solid phase synthesis. These positively charged oligonucleotides are defined as molecular conjugates whose size is close to that of oligonucleotides - a few nanometers. Such cationic oligonucleotides are able to bind the cell membrane, inducing an endocytosis and transport in the cytoplasm.6 The study of the influence of the global charge of the conjugate (spermine/phosphate ratio) showed carrier-free intracellular delivery for oligonucleotide conjugates having a net positive charge (see Figure 4, Page 4). In addition, when compared to cationic lipid mediated delivery, a diffuse cytoplasmic pattern of cationic oligonucleotides indicates an efficient access to the cytoplasm as molecules and not as aggregates. This observation suggests an endosome escape of cationic oligonucleotides based on the proton sponge mechanism associated with the individual amine pK’s of the oligospermine moieties.
Figure 4: Self-delivering cationic oligonucletides, antisense (Left) and siRNA (Right). |
|
 |
 |
Intracellular delivery of 5’-Fluo-N19 (upper) and 5’-Fluo-Z18-N19 (lower) in HeLa after 2 h incubation at 2 µM and 500 nM, respectively. |
Intracellular delivery of 5’-Rho-siRNA with cationic lipid INTERFERin (upper) and 5’-Rho-Z25-siRNA in A549 cells after 24 h incubation at 10 nM and 100 nM, respectively. |
Activity results have also confirmed the requirement for an overall positive charge of the conjugated siRNA (Z25 to Z35 conjugates) in many cellular models and for many targets.7 A specific gene silencing was obtained at sub-micromolar concentration (25 to 100 nM) in the presence of serum and the RNAi mechanism was confirmed by a 5’-RACE assay as well as the use of control siRNAs.
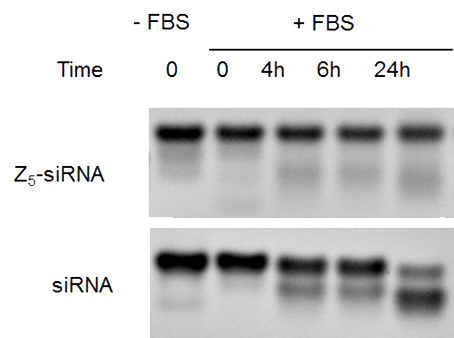
More importantly, the oligospermine tail protects the cationic oligonucleotide from degradation. This was observed with conjugates containing a low number of spermines (Z5-siRNA) where a limited degradation was shown even after 24 h incubation in serum, contrasting with unmodified siRNAs which were significantly degraded (Figure 5). Self-delivering and biologically active phosphodiester-based siRNAs containing 30 spermine moieties were also shown to be highly stable in presence of serum.7 The spermine tail effect against the siRNA degradation was also confirmed in an RNase A assay (Figure 5). In these examples of siRNA conjugates, the cationic tail was grafted on the 5’-end of sense strands but it is expected that a spermine tail at either end of the oligonucleotide will improve the nuclease stability.
Figure 5: The oligospermine tail protects the siRNA conjugates from degradation |
|
 |
 |
Analysis on Tris-Tricine polyacrylamide 10−20% gel
|
Analysis on SDS−Tris-Tricine polyacrylamide 10−20% gel
|
By selecting the number of cationic units, the global charge of the ZNA molecules can be modulated which defines their field of applications. When negatively charged, ZNA are potent tools for molecular biology and diagnostic applications. Their design is essentially based on the expected and predictable Tm of the oligonucleotide which depends on the number of conjugated cationic units. When positively charged, the cationic conjugates become self-delivering oligonucleotides into cells and resistant to nucleases which make them very attractive molecules for antisense or RNA interference applications.
"Spermine phosphoramidite" synthon is the subject matter of U.S. Divisional Patent Application No. 14/745,871, European Patent No. 1 973 927 and foreign equivalents for which Polyplus-transfection is the co-owner. Product is sold for research purposes only.
Product shall not be used to manufacture oligonucleotide-oligospermine conjugates for use in diagnostics, clinical or commercial applications including use in humans. There is no implied license to manufacture oligospermine-oligonucleotide conjugates for diagnostic, clinical or commercial applications, including but not limited to contract research. For a commercial license to Polyplus-transfection Intellectual Property, please visit the Polyplus-transfection webpage dedicated to Licensing Opportunities.
We would like to take this opportunity to thank our colleagues at Polyplus for preparing this article and are delighted to share it with our readers. This report updates the research described in a previous Glen Report.3
Glen Research has been able to offer Spermine Phosphoramidite (1) to our customer base wordwide, under licence from Polyplus, since 2012. Over the intervening period, we have been able to help our research customers to simply and reliably prepare oligospermine 5'-conjugates of the structure shown in Figure 7 and also equivalent structures at the 3'-terminus. We certainly expect that publication of this update in this Glen Report will demonstrate the utility of spermine technology in an even broader array of research endeavors.

1. B. Pons, M. Kotera, G. Zuber, and J.P. Behr, Chembiochem, 2006, 7, 1173-6.
2. E. Voirin, J.-P. Behr, and M. Kotera, Nat. Protocols, 2007, 2, 1360-1367.
3. The Glen Report, 2012, 24.1, 1-4.
4. V. Moreau, et al., Nucleic Acids Res, 2009, 37, e130.
5. C. Paris, et al., Nucleic Acids Res, 2010, 38, E95-E95.
6. M. Nothisen, M. Kotera, E. Voirin, J.S. Remy, and J.P. Behr, J Amer Chem Soc, 2009, 131, 17730-1.
7. C. Paris, et al., Mol Pharm, 2012, 9, 3464-75.
8. R. Noir, M. Kotera, B. Pons, J.S. Remy, and J.P. Behr, J Amer Chem Soc, 2008, 130, 13500-13505.
9. H. Kandemir, et al., Neurosci Lett, 2014, 580, 158-62.
10. Z. Iranmanesh, et al., Asian Pac J Cancer Prev, 2015, 16, 1919-24.
11. B. Alaiyan, et al., BMC Cancer, 2013, 13, 196.
12. K.T. Gagnon, et al., J Amer Chem Soc, 2011, 133, 8404-7.