Glen Report 9.12: Protecting Groups for DNA, RNA and 2'-OMe-RNA Monomers
RNA Monomers
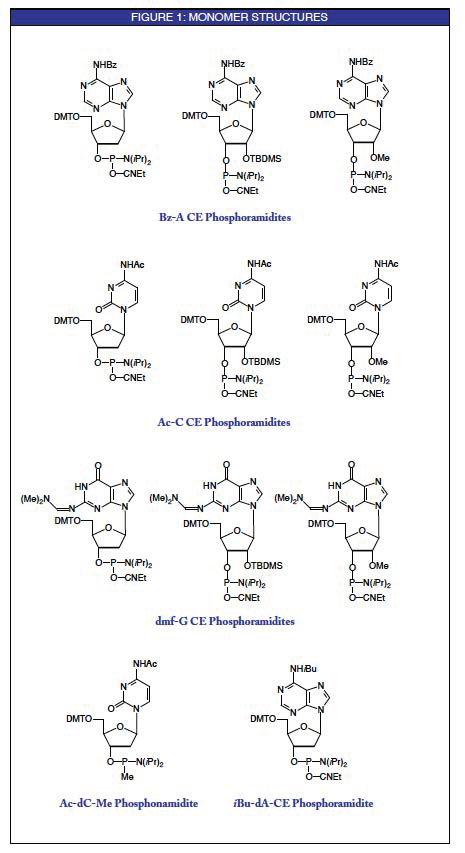
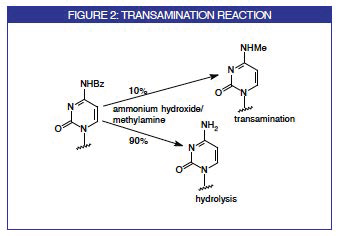
The use of acetyl (Ac) protecting groups on cytosine residues allows deprotection with a variety of strong organic bases instead of or in combination with ammonia. This is a consequence of the fact that the hydrolysis of the acetyl group occurs virtually instantly and the competing transamination reaction, described in Figure 2 using benzoyl (Bz) as an example, with the organic amine is eliminated.1 This process which is in routine use for DNA synthesis has now become popular for RNA synthesis.2 In the deprotection procedure using a 50:50 aqueous methylamine : ammonium hydroxide mix (AMA), the oligonucleotide made with the Ac-C monomer is cleaved and deprotected at 65° for 10 minutes. This occurs regardless (within reason) of the nature of the protecting groups on the A and G monomers. Glen Research is now offering A and G RNA monomers with benzoyl (Bz) and dimethylformamidine protecting groups, respectively. These monomers are offered in addition to our phenoxyacetyl (PAC) protected A and G monomers which are more versatile and allow a variety of very mild deprotection schemes to be used.
DNA and 2'-OMe-RNA Monomers
The updated set of base protecting groups we have chosen for RNA monomers (Bz-A, Ac-C and dmf-G) is already available for DNA and 2'-OMe-RNA monomers. For those researchers making chimeric oligos, this makes the deprotection more straightforward.
Methyl Phosphonamidites
The methyl phosphonate backbone may be used to induce nuclease resistance into oligonucleotides prepared for antisense research. Because the backbone is very base labile, these oligos are traditionally deprotected with ethylene diamine in ethanol. In cooperation with Beckman Instruments, we can now offer Ac-dC methyl phosphonamidite. Using Bz-dC methyl phosphonamidite and deprotecting with ethylene diamine, the level of transamination has been measured to be of the order of 16%. Using the more labile isobutyryl (iBu) protecting group, the level of transamination is much reduced at around 4%. However, using the Ac-dC monomer with ethylene diamine, the level of transamination is lowered to an undetectable level.3
Photochemical Cleavage Reactions
Photolabile supports are becoming popular for the preparation of 3'-modified oligonucleotides which can be released into solution with all protecting groups intact (see Page 6). Aromatic protecting groups like benzoyl absorb some of the photochemical energy during cleavage and, therefore, should be avoided.4,5 We now offer isobutyryl protected dA (iBu-dA) monomer to be used in conjunction with Ac-dC and dmf-dG. These monomers provide a set of protecting groups with the appropriate photochemical characteristics.
Gas Phase Deprotection
One of the most significant advances in deprotection has been described recently.6 In this novel procedure, anhydrous ammonia gas is used to effect cleavage and deprotection safely and conveniently in parallel on as many columns as will fit in a reactor. Since no water is present, the fully-deprotected oligonucleotides remain adsorbed to the column matrix, guaranteeing no cross-contamination. The oligonucleotides can then be eluted with water and desalted or further purified, if desired. Using PAC-protected monomers, the cleavage and deprotection processes can be completed in 36 minutes.
References
(1) M.P. Reddy, N.B. Hanna, and F. Farooqui, Tetrahedron Lett., 1994, 35, 4311-4314.
(2) M.P. Reddy, F. Farooqui, and N.B. Hanna, Tetrahedron Lett., 1995, 36, 8929-8932.
(3) M.P. Reddy, F. Farooqui, and N.B. Hanna, Tetrahedron Lett., 1996, 37, 8691-8694.
(4) D.J. Yoo and M.M. Greenberg, J. Org. Chem., 1995, 60, 3358-3364.
(5) D.L. McMinn and M.M. Greenberg, Tetrahedron, 1996, 52, 3827-3840.
(6) J.H. Boal, A. Wilk, N. Harindranath, E.E. Max, T. Kempe, and S.L. Beaucage, Nucleic Acids Res., 1996, 24, 3115-3117.
Product Information
Natural RNA Phosphoramidites and Supports
iBu-dA-CE Phosphoramidite (10-1009-xx) has been discontinued
- Glen Report 9.11: A Novel Transfection Reagent
- Glen Report 9.12: Protecting Groups for DNA, RNA and 2'-OMe-RNA Monomers
- Glen Report 9.13: A Gallery of Recent Additions
- Glen Report 9.14: 3'-Labelling - Dabcyl CPG, Photolabile 3'-Amino-CPG
- Glen Report 9.15: Properties of Oligonucleotides Containing the Bases P and K
- Glen Report 9.16: Non-Aqueous Oxidation with 10-Camphorsulfonyl-Oxaziridine
- Glen Report 9.17: Cyclic Oligonucleotides
- Glen Report 9.18: DNA Synthesis Columns

