Simple and efficient labelling of oligonucleotides with a variety of labels is now routine through Click Chemistry.1 Earlier Glen Reports have highlighted several approaches for efficient labelling of oligonucleotides with Click Chemistry.2-6 All of our alkyne modifiers have proved to be excellent substrates for Click Chemistry in the presence of the water-soluble Click ligand, THPTA.
1-Ethynyl-dSpacer CE Phosphoramidite (1) is a new alkyne modifier that has the potential for producing and studying site-specific DNA inter-strand crosslinks. This modifier is stable, possesses high coupling efficiency, retains the standard phosphodiester backbone, is stable to standard deprotection techniques, and is an excellent substrate for Click Chemistry.
In the previous Glen Report,4 we described the use of 1-Ethynyl-dSpacer. We reported on the Click reaction of this modifier with a variety of azides to form a selection of 1,2,3-triazole pseudo-nucleoside analogues of general structure as shown in Figure 1. There are undoubtedly many potential uses for these labelled pseudo-nucleosides but, in the present Technical Brief, we highlight our experience with oligonucleotides modified with psoralen and coumarin and their potential for studying inter-strand crosslinks (ICL).

Naturally occurring ICL originate from a variety of exogenous and endogenous sources that include alkylating agents, ionizing radiation, metabolism and environmental exposure.7 Cellular repair of ICL has been linked to an assortment of DNA repair enzymes. Genomic defects associated with ICL are implicated in cancer and confer hypersensitivity to cross-linking agents. A cost effective path for generating ICL is essential for studying these repair mechanisms. There are many approaches for generating ICL including non-specific agents as well as site-specific approaches.8-11
In this Brief, we describe the use of 1-Ethynyl-dSpacer for the site-specific incorporation of 4’-azidomethyl-4,5,8-trimethylpsoralen (2) or 7-azido-4-methyl coumarin (3) as possible approaches for generating photoinducible internal and terminal ICL. Psoralen and coumarin undergo photo-induced cycloaddition with pyrimidine nucleosides on exposure to long wavelength UV light (300-350nm).12,13 In some cases, these ICL are reversible with short wavelength UV light (254nm).
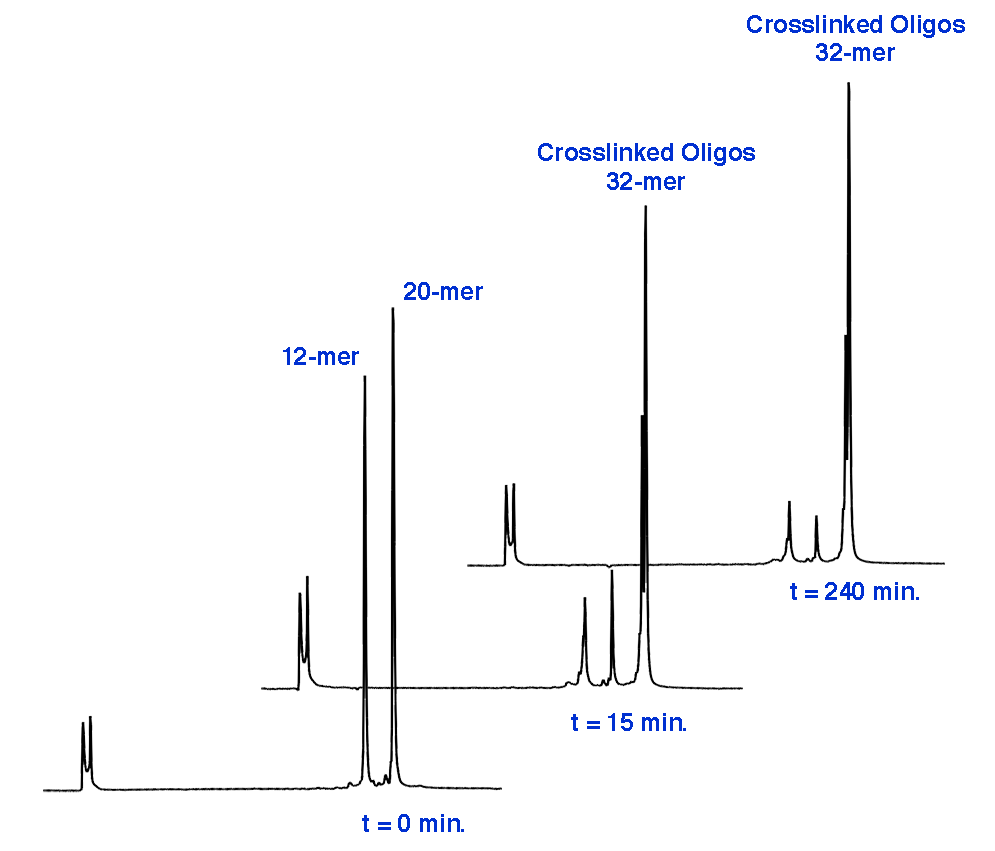
In this set of experiments, we synthesized a group of test oligonucleotides incorporating 1-Ethynyl-dSpacer (12-mer) and a set of complementary target oligonucleotides (20-mer) (Table 1). All oligonucleotides were purified DMT-ON using standard Glen-Pak protocols. The test oligonucleotides included site-specific internal modification or a 3’-terminus modification. Each test oligonucleotide containing 1-Ethynyl-dSpacer was conjugated with high efficiency to either the psoralen azide (2) or the coumarin azide (3) via standard Click Chemistry and purified by simple ethanol precipitation. Representative chromatograms with attendant UV spectra of the products are shown in Figure 2.


The test and target oligonucleotides were hybridized to form the duplexes shown in Table 1. Each duplex was exposed to long wavelength light (365nm) from a handheld UV lamp for up to 4 hours at room temperature to generate cross-linked oligonucleotides. The formation of ICL was evaluated using a denaturing IEX HPLC method capable of resolving each oligo as well as the cross-linked duplex. Duplexes 1 and 5 were evaluated for reversal of crosslinking by exposure to short wavelength UV light.
3’-G CTG AXT GAT AT-5’
5’-TAA TAC GAC TCA CTA TAG GG 3’
3’-G CTG XGT GAT AT-5’
5’-TAA TAC GAC TCA CTA TAG GG 3’
3’-G CTG XGT GAT AT-5’
5’-TAA TAC GAC TAA CTA TAG GG 3’
3’-G CTG AXT GAT AT-5’
5’-TAA TAC GAC TAA CTA TAG GG 3’
3’-G CTG AGT GAT AX-5’
5’-TAA TAC GAC TCA CTA TAG GG 3’
3’-G CTG XGT GAT AT-5’
5’-TAA TAC GAC TCA CTA TAG GG 3’
3’-G CTG AXT GAT AT-5’
5’-TAA TAC GAC TCA CTA TAG GG 3’
3’-G CTG AGT GAT AX-5’
5’-TAA TAC GAC TCA CTA TAG GG 3’
The level and rate of crosslinking varied depending on the location of the modification (Table 2). Some crosslinking was observed in as little as 15 minutes for all duplexes although most duplexes required significantly longer exposure time for maximum yield. As modifiers for oligonucleotide synthesis, psoralen labels are typically added to the 5’-terminus with the canonical target sequence as 5’-TpA-3’.13 Surprisingly, the best result (Duplex 1) with Psoralen was obtained with the internal sequence of 5’-CpT-3’ and 1-Ethynyl-dSpacer opposite dC, resulting in 77% ICL formation in as little as 15 minutes. Overall ICL yield rose to 87% after 2 hours with a maximum of 89% after a 4-hour exposure. In comparison, when psoralen was incorporated opposite dT (Duplex 2), the initial rate was reduced and the duplex required 4 hours for a maximum yield of 81%. Despite the low initial rates when opposite dT, high final yields for both duplexes were still observed and are most likely a result of two potential nucleoside targets (dT and dC) for crosslinking with Psoralen.
Modification Duplex |
Site |
Opposite |
15 min. |
60 min. |
120 min. |
240 min. |
|
Psoralen Duplex 1 |
Internal |
TC |
77% |
87% |
87% |
89% |
|
Psoralen Duplex 2 |
Internal |
TC |
10% |
51% |
68% |
81% |
|
Psoralen Duplex 3 |
Internal |
TA |
22% |
50% |
62% |
64% |
|
Psoralen Duplex 4 |
Internal |
TA |
52% |
68% |
72% |
76% |
|
Psoralen Duplex 5 |
Terminal |
TA |
77% |
78% |
78% |
74% |
|
Coumarin Duplex 6 |
Internal |
TC |
3% |
10% |
17% |
26% |
|
Coumarin Duplex 7 |
Internal |
TC |
3% |
5% |
14% |
25% |
|
Coumarin Duplex 8 |
Terminal |
TA |
8% |
23% |
40% |
58% |
After substituting dC with dA in the target sequence (Duplexes 3, 4), the relative initial rates were reduced to 22% and 52% at 15 minutes compared to the ideal sequence 5’-TpC-3’ (Duplex 1). The reduced initial yields may result from the removal of a potential crosslink with cytosine.
With a terminal 1-Ethynyl-dSpacer modifier, the psoralen was incorporated opposite dA in the terminal sequence 5’-TpA-3’ (Duplex 5). A 15 minute exposure resulted in 77% ICL formation. Prolonged exposure of 4 hours, however, resulted in reduced final yields suggesting additional side reactions of psoralen. Indeed, in the absence of target, psoralen-containing oligonucleotides were rapidly degraded. Terminal modifications may provide steric freedom from unwanted side reactions. In contrast, internal incorporations may be shielded through base stacking that prevents subsequent side reactions.
Psoralen crosslinks are often reversible after exposure to short wavelength UV light (254nm). Duplexes 1 and 5 were evaluated for potential reversal for up to 4 hours. Some reversal, 19% and 43% respectively, was observed after exposure and prolonged exposure times (>4 hours) may be required.
Coumarin conjugated oligonucleotides also formed ICL after exposure to long wavelength UV light (Duplexes 1, 2, 3). The initial and final ICL rates were significantly lower than psoralen-containing oligonucleotides. Interestingly, the coumarin-modified duplexes generated a less complex HPLC profile suggesting the formation of a single product. Extending the overall exposure time should result in a more extensive conversion.
1-Ethynyl-dSpacer, in combination with psoralen and coumarin azides, has been shown to be a potential research tool for studying ICL. As a simple Click tool, 1-Ethynyl-dSpacer works exceptionally well to introduce a 1,2,3-substituted-triazole residue into an oligonucleotide as a pseudo nucleobase, while preserving the natural phosphodiester backbone. With the selection of a suitable azide such as psoralen or coumarin, the modifier can be used to incorporate photo-inducible and site-specific ICL. The versatility of 1-Ethynyl-dSpacer is highlighted by the fact that a single 1 µmole synthesis can be used with multiple azides to generate and evaluate several different structures.
1. Q. Wang, et al., Journal of the American Chemical Society, 2003, 125, 3192-3193.
2. The Glen Report, 2010, 22.1, 1-4.
3. The Glen Report, 2011, 23.1, 4, 12.
4. The Glen Report, 2015, 27.2, 1-3.
5. The Glen Report, 2007, 19.1, 9.
6. The Glen Report, 2014, 26.2, 7-8.
7. C. Clauson, O.D. Schärer, and L. Niedernhofer, Cold Spring Harbor Perspectives in Biology, 2013, 5.
8. S. Hentschel, J. Alzeer, T. Angelov, O.D. Scharer, and N.W. Luedtke, Angewandte Chemie-International Edition, 2012, 51, 3466-3469.
9. S. Ghosh, and M.M. Greenberg, Journal of Organic Chemistry, 2014, 79, 5948-5957.
10. P.P. Christov, K.J. Son, and C.J. Rizzo, Chemical Research in Toxicology, 2014, 27, 1610-1618.
11. Y. Yoshimura, and K. Fujimoto, Org Lett, 2008, 10, 3227-30.
12. H.B. Sun, H.L. Fan, and X.H. Peng, Journal of Organic Chemistry, 2014, 79, 11359-11369.
13. U. Pieles, and U. Englisch, Nucleic Acids Res., 1989, 17, 285.