P.L. Bartels and J.K. Barton
Division of Chemistry and Chemical Engineering
California Institute of Technology
Pasadena, CA 91125
DNA is traditionally thought of as the cell's library of genetic information. However, work in the Barton lab over the last three decades has brought to light a new side of DNA, revealing the ability of this molecule to facilitate the transport of charge over long molecular distances. This fascinating property of DNA has been investigated under biologically relevant conditions using a variety of redox probes.
|
A  |
 |
 |
|
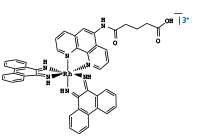
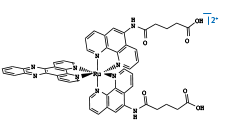
| Figure 1: DNA binding by Intercalating Metal Complexes Fluorescent [Ru(phen')2(dppz)]2+ and [Rh(phi)2(phen')]3+ quencher covalently tethered to a 15-mer DNA duplex. A) Intercalated metal complexes, with Rh (III) at top and Ru (II) at bottom. B ) Free [Rh(phi)2(phen')]3+. C) Free [Ru(phen')2(dppz)]2+. C.J. Murphy, M.R. Arkin, Y. Jenkins, N.D. Ghatlia, S.H. Bossmann, N.J. Turro, and J.K. Barton Science (1993) 262 p. 1025. |
|


One of the first studies to examine DNA-mediated charge transport utilized covalently bound metallointercalators to explore long range quenching of luminescence by DNA-mediated electron transfer. [Ru(phen')2dppz)]2+ and [Rh(phi)2(phen')]3+ were covalently tethered to opposite ends of a 15-mer DNA duplex1. Here covalent tethering made electron transfer through the π-stacked base pairs the only pathway fully able to explain the observed luminescence quenching. This experiment really provided the first indication that long range electron transfer could proceed through the DNA duplex.
While the above studies were able to establish the occurrence of DNA-mediated charge transport in excited state systems, a different method was required to investigate these processes in the ground state. Electrochemical techniques provided the ideal means of carrying out this aim. In these experiments, an organic redox probe was tethered to one end of a DNA strand which was then annealed to an alkanethiol-modified complement. The thiol linker facilitated the formation of self-assembled DNA monolayers on a gold electrode, allowing direct interrogation of electron transfer between the surface and the probe.
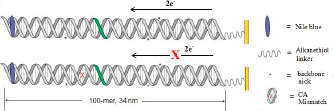
Using cyclic voltammetry with a Nile Blue reporter covalently bound and well coupled electronically to the DNA, a key set of experiments compared electron transfer rates between a 100-mer and a 17-mer DNA duplex2. In fact, ground state electron transfer could proceed through the 100-mer duplex to reduce the covalently bound Nile Blue. Moreover, electron transfer rates were virtually indistinguishable, showing almost no attenuation over 100 base pairs (a distance of 34 nm). Remarkably, the presence of multiple nicks in the phosphate backbone had no effect on this process, while the incorporation of a single mismatched base pair significantly attenuated the signal, revealing that long-range charge transfer through DNA is dependent on base-pair stacking alone. Interestingly, the attenuation associated with the presence of the single base mismatch was equivalent for the 100-mer and for the 17-mer. Finally, the probe signal could be removed by reaction with a blunt end restriction enzyme, showing that surface-bound DNA exists in a biologically accessible conformation and paving the way for further applications of electrochemistry with DNA.
While the work with Nile Blue made great progress in establishing the general mechanisms of DNA charge transport, the short tether length of Nile Blue does not permit intercalation into the base stack, resulting in relatively small signals. This issue was resolved by the use of methylene blue covalently bound to amino-modified DNA. Covalent methylene blue exhibits the same desirable electron transfer properties as Nile Blue, but the flexible linker allows the probe to directly intercalate into the base stack, improving charge transfer signal size. An additional degree of versatility with this probe can be achieved by varying the surface density of the DNA monolayer on gold, favoring DNA-mediated (high density) or surface-mediated (low density) processes3, thus making covalent methylene blue suitable for a wide range of diagnostic applications.
Building on this body of earlier work, DNA-mediated charge transport has seen widespread applicability in areas ranging from analyte detection to biochemistry. Organic probes have been particularly useful in these pursuits, as demonstrated by the recent use of covalent methylene blue as a reporter of human Dnmt1 methyltranferase activity4 (known to be aberrant in several cancer types). Tethered metal complexes have been applied in novel ways in recent work, as exemplified by a study which used photoexcited [Ru(phen)(dppz)(bpy')]2+ to oxidize guanines in DNA (a process requiring potentials too high to observe on gold electrodes) with or without a bacterial ferritin, allowing the capability of this protein to prevent oxidative DNA damage in bacterial pathogens to be assessed5.
As the examples above have shown, the applications of DNA-mediated charge transport have expanded dramatically in recent years, a trend which shows no signs of slowing. The addition of methylene blue and ferrocene phosphoramidites to the Glen Research catalogue at this time is thus particularly exciting, and will no doubt contribute significantly to the continued advancement of this field.

See Also: New Product - Methylene Blue NHS Ester