Glen Report 17.12: Products for siRNA Research
Amino-Modifier C6-U
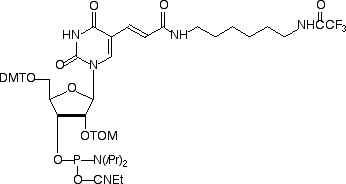
Amino-Modifier C6-U
Amino-Modifier C6-dT was first described1,2 in the mid 1980s when interest in labeling oligos was very limited. The original usage was to attach alkaline phosphatase to oligos for diagnostic applications. The molecule was set up perfectly for this kind of use since the linker arm projects into the major groove of double-stranded DNA where there is room for large molecules without disruption of hybridization.3 Over the years, we have added products to our range based on amino-modifier C6-dT labeled with Biotin, Dabcyl, TAMRA, Fluorescein and, in this issue, BHQ-1™ and BHQ-2™. Now it is time to add the RNA analogue Amino-Modifier C6-U. Initially, we have chosen the popular TOM group4 for protection of the 2’-hydroxyl. We welcome Amino-Modifier C6-U to this growing structural family and envisage applications in RNA structural studies as well as for labeling siRNA to probe uptake and cellular distribution.
References:
- J.L. Ruth, C. Morgan, and A. Pasko, DNA, 1985, 4, 93.
- E. Jablonski, E.W. Moomaw, R.H. Tullis, and J.L. Ruth, Nucleic Acids Res., 1986, 14, 6115.
Amino-Modifier C6-U (cont.)
- J. Telser, K.A. Cruickshank, L.E. Morrison, and T.L. Netzel, J. Am. Chem. Soc., 1989, 111, 6966-6976.
- S. Pitsch, P.A. Weiss, L. Jenny, A. Stutz, and X.L. Wu, Helv Chim Acta, 2001, 84, 3773-3795.
Product Information
Amino-Modifier C6-U Phosphoramidite (10-3039)
6-Thio-G
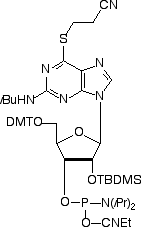
Nucleotides with sulfur substituting for oxygen at the 4-position of pyrimidines and the 6-position of purines have proved to be very useful in molecular biology. In particular, the sulfur is active photochemically and available to photo-crosslink to an adjacent molecule, allowing study of internucleotide and nucleotide-protein interactions. Because the sulfur is at a position actively used for hydrogen bond interactions, the distance to the complementary target is short and cross-linking occurs specifically, allowing internucleotide interactions to be explicitly defined. Sulfur analogues of 2’-deoxynucleosides have been available as phosphoramidites for a long time and 4-thiouridine (4-thio-U) in the RNA series has been available also. We now add 6-thioguanosine (6-thio-G) to the tools available for studying RNA-RNA and RNA-protein interactions by offering 6-thio-G phosphoramidite for incorporation into oligoribonucleotides.
There have been several reports1-3 in the literature describing 6-thio-G phosphoramidite but it is only recently that the demand for minor RNA phosphoramidites has made this feasible as a product. It is easy to envisage applications for this product in ribozyme and siRNA applications, as well as in RNA-protein interactions.
The removal of the silyl protecting group without interfering with the sulfur is critical, so we have used the more traditional t-butyldimethylsilyl protecting group on the 2’-hydroxyl. This is removed1 cleanly by triethylamine trihydrofluoride in DMSO but t-butylammonium fluoride (TBAF) leads to degradation of the thio-nucleotide analogue and should not be used.
References
- C.J. Adams, J.B. Murray, M.A. Farrow, J.R.P. Arnold, and P.G. Stockley, Tetrahedron Lett., 1995, 36, 5421-5424.
- Y. Wang, E. Lattmann, and Q. Zheng, Nucleos Nucleot Nucleic Acids, 2003, 22, 1247-1249.
- Q.G. Zheng, Y. Wang, and E. Lattmann, Bioorg Medicinal Chem Letter, 2003, 13, 3141-3144.
Product Information
6-Thio-G-CE Phosphoramidite (10-3072)
3-Deaza-dA
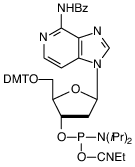
3-Deaza-dA
Modified base analogues of 2’-deoxynucleosides are readily available for probing interactions in the major groove of duplex DNA. However, there are far fewer analogues available to investigate interactions in the minor groove. The standard nucleobases have an unshared pair of electrons that project into the minor groove of duplex DNA. In the case of the purines, this is the nitrogen at N3 and, for the pyrimidines, it is the keto group at C2. Enzymes that interact with DNA, polymerases, reverse transcriptases, restriction enzymes, etc., may use a hydrogen bond donating group to contact the hydrogen bond acceptor in the minor groove. 3-Deaza-2’-deoxyadenosine is very interesting in that it maintains the ability for regular Watson-Crick hydrogen bonding to T but is lacking the electron pair at the 3-position normally provided by N3. A very interesting recent publication from the Benner group describes1 using 3-deaza-2’-deoxyadenosine to probe minor groove contacts by polymerases and reverse transcriptases in the context of biological evolution.
An earlier paper2 discussed the thermodynamic stability of oligonucleotides containing 3-deaza-2’-deoxyadenosine. Surprisingly, substitution of 3-deaza-2’-deoxyadenosine for 2’-deoxyadensine lowered duplex stability substantially by around 4° per insertion. The authors surmised that this was due to the higher pKa (6.80) of 3-deaza-2’-deoxyadenosine in comparison to 2’-deoxyadenosine (3.62), which allowed protonation of the base, and the loss of stabilizing hydration of the minor groove electron pair.
We have had a long-term interest in supplying the phosphoramidite of 3-deaza-2’-deoxyadenosine, which was very challenging3,4 to prepare in quantity. We are delighted to offer this phosphoramidite as a result of the perseverance of our colleagues at Berry and Associates, Inc.
References
- C.L. Hendrickson, K.G. Devine, and S.A. Benner, Nucleic Acids Res., 2004, 32, 2241-50.
- C. Lever, X. Li, R. Cosstick, S. Ebel, and T. Brown, Nucleic Acids Res., 1993, 21, 1743-1746.
- R. Cosstick, X. Li, D.K. Tuli, D.M. Williams, B.A. Connolly, and P.C. Newman, Nucleic Acids Res., 1990, 18, 4771.
- F. Seela, T. Grein, and S. Samnick, Helvetica Chimica Acta, 1992, 75, 1639-1650.
Product Information
3-deaza-dA-CE Phosphoramidite (10-1088)
C-5 propynyl Pyrimidine Analogues
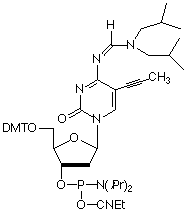

Substitution of C-5 propynyl-dC (pdC) for dC and C-5 propynyl-dU (pdU) for dT1 are effective strategies to enhance base pairing. This increase in hybridization efficiency is due to the hydrophobic nature of the groups at the C-5 position which helps to exclude water molecules from the duplex.
Using these base substitutions, duplex stability and therefore melting temperatures are raised by the approximate amounts shown:
| Base | Tm increase |
|---|---|
| C-5 propynyl-C | 2.8° per substitution |
| C-5 propynyl-U | 1.7° per substitution |
While these modifications have found most applications in antisense oligonucleotides, their ability to enhance binding while maintaining specificity will also prove useful in the synthesis of high affinity probes.
We have completed an agreement with Isis Pharmaceuticals, Inc. to recommence the supply of these two valuable products. We believe these will be a welcome addition to the selection of modified bases that affect hybridization.
Users of oligos containing pdC and pdU must take heed of the following statement:
This product is covered by patents or patents pending owned by Isis Pharmaceuticals, Inc. ("Isis"). Purchase of this product includes a limited license to use this product solely for internal research. This license specifically excludes (and you have no right to use this product for): (a) therapeutic or diagnostic applications (including products or services that incorporate this product), (b) any in vivo toxicity/safety study in support of an investigational new drug application (or foreign counterpart), (c) resale (including sale of any products or services that incorporate this product) or (d) gene functionalization activities (including products or services that incorporate data derived from gene functionalization activities) if such activities have commercial application, any and all of which require a separate license from Isis. Neither this product nor any product created through its use may be used in human clinical trials.
At the time of this writing, in the event you have a separate agreement with Isis regarding this product which explicitly states that the foregoing is not applicable to you, your use of this product will be governed by the terms of such agreement. In no event does the limited license included with the purchase of this product expand or alter the scope of the license granted pursuant to such agreement.
References
- B.C. Froehler, S. Wadwani, T.J. Terhorst, and S.R. Gerrard, Tetrahedron Lett., 1992, 33, 5307-5310.
Product Information
pdC-CE Phosphoramidite (10-1014)
pdU-CE Phosphoramidite (10-1054)
Pyrrolo-CTP


Pyrrolo-dC is a fluorescent nucleoside that codes as dC and base pairs efficiently with dG. We have published a preliminary report1on the chemistry of pyrrolo-dC and further details of the chemistry and biology are currently in press. Preliminary evidence indicates that pyrrolo-dC triphosphate is an excellent substrate for Taq, Pfu and Vent polymerases and is incorporated specifically opposite dG. Pyrrolo-dCTP has been available for some time and is in use in biological assays. We are now introducing pyrrolo-CTP, the ribonucleoside triphosphate. We anticipate that the addition of a fluorescent ribonucleotide with fluorescence exquisitely sensitive to its environment would be of great interest for RNA structural research.
The pyrrolo-C project is a joint development by Berry and Associates and Glen Research. Patents covering this modified fluorescent base and its uses are currently pending.
References
- D.A. Berry, et al., Tetrahedron Lett, 2004, 45, 2457-2461.
Product Information
Pyrrolo-dCTP (10mM) (81-1017) has been discontinued.
Pyrrolo-CTP (10mM) (81-3017) has been discontinued.
Pyrrolo-dC-CE Phosphoramidite (10-1017)
Pyrrolo-C-TOM-CE Phosphoramidite (10-3017)
- Glen Report 17.11: Introduction
- Glen Report 17.12: Products for siRNA Research
- Glen Report 17.13: UniCap Phosphoramidite, AN alternative to acetic anhydride capping
- Glen Report 17.14: Expanding Our Repertoire of Dark Quenchers: Black Hole Quenchers
- Glen Report 17.15: 2'-Fluoro-RNA Monomers
- Glen Report 17.16: Internally Quenched Nucleotide Fluorescent Reporters
- Glen Report 17.17: AB 3900 and MerMade Columns

