Glen Report 17.14: Expanding Our Repertoire of Dark Quenchers: Black Hole Quenchers
Introduction
Fluorescence Resonance Energy Transfer (FRET) has become one of the most popular tools to assay nucleic acids. This is because FRET lends itself to high throughput automation and is quite sensitive, making it the method of choice for sequence and single nucleotide polymorphism (SNP) analysis. In addition, it is highly useful for probing DNA and RNA structure, dynamics and intermolecular interactions.
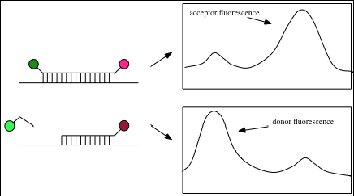
The basis of FRET is the dipole-dipole coupling of donor and acceptor molecules in which the energy of the donor in the excited state is transferred to the acceptor molecule. The efficiency of the energy transfer depends upon a variety of factors – the distance between the donor and acceptor molecules and their orientation, the quantum yield of fluorescence of the donor, the extinction coefficient of the acceptor and the spectral overlap between the emission of the donor and the absorbance of the acceptor.1 In a traditional FRET experiment, both the acceptor and donor molecules are fluorophores, with the 5’ terminus labeled with the donor and the 3’ with the acceptor. Upon excitation of the donor, the acceptor fluoresces and the donor is quenched. If, however, the donor/acceptor pair is separated by a conformational change or action such as cleavage by a nuclease, the donor fluorescence is unaffected, as shown in Figure 1.
Dark Quenchers
The quencher need not be a fluorophore, however. A non-fluorescent chromophore can be used that overlaps with the donor’s emission (a dark quencher). In such a case, the transferred energy is dissipated as heat.
FRET probes that utilize dark quenchers have a number of advantages over their fluorophore-labeled counterparts. They exhibit lower background fluorescence which leads to a larger signal-to-noise ratio, and, therefore, greater dynamic range.2 In addition, since there is no secondary fluorescence arising from a dark quencher, multiple fluorophores can be simultaneously spectrally resolved, making dark quencher probes amenable to multiplex assays. But one of the most endearing qualities of a FRET probe designed with a dark quencher is the ease of synthesis; dark quenchers are generally more robust than their fluorescent counterparts and resist degradation during oligonucleotide deprotection. As a result, the more expensive UltraMild monomers are not required. And as an added bonus, because the failure sequences are non-fluorescent, dark quencher probes are not plagued by high background fluorescence with even unpurified probes.
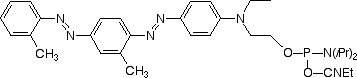
One of the first reported 'dark quenchers' was the azobenzene dye Dabcyl.3 With a broad absorbance centered around 478 nm, Dabcyl was ideal for quenching dyes by FRET that fluoresce in the blue to green region, such as EDANS. However, its spectral overlap with one of the most prevalent dyes, fluorescein, was not optimal. So, in 2002, Glen Research added the Eclipse™ Quencher from Epoch Biosciences to its product line. With a maximum absorbance at 522 nm, it was ideally suited to quench fluorescein and did so at 96% efficiency.4
Black Hole Quencher Dyes
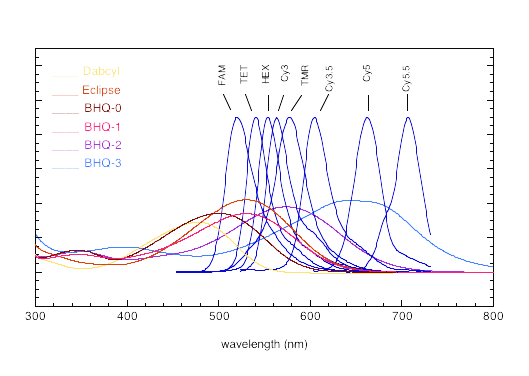
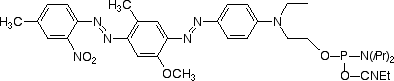
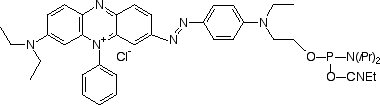
In keeping with the growing popularity of red and near-infrared dyes, we are expanding our dark quencher line further. We are, therefore, happy to provide in collaboration with Biosearch Technologies, the Black Hole Quencher™ dyes (BHQs), whose physical properties are detailed in Table 1. The BHQ dyes are robust dark quenchers that very nicely complement our existing product line. They are compatible with ammonium hydroxide deprotection, exhibit excellent coupling efficiencies, have large extinction coefficients and are completely non-fluorescent. Their absorbances are well-tuned to quench a variety of popular fluorophores – even those far into the red, such as Cy3 and Cy5 (Figure 2).
| Quencher | λmax (nm) | E260 (L/mol•cm) | Emax (L/mol•cm) |
|---|---|---|---|
| BHQ-0 | 493 | 7,700 | 34,000 |
| BHQ-1 | 534 | 8,000 | 34,000 |
| BHQ-2 | 579 | 8,000 | 38,000 |
| BHQ-3 | 672 | 13,000 | 42,700 |
However, FRET is not the only means by which a fluorophore can be quenched. Another mechanism is static quenching due to the formation of a non-fluorescent ground-state complex. The complex is stabilized by induced-dipole and hydrophobic interactions and its formation is characterized by a decrease in the monomeric dye absorption band and an increase in a blue-shifted, non-fluorescent band. Static quenching is utilized in Molecular Beacons, in which a dark quencher is held in close proximity to the fluorophore in a hairpin stem.5 The dark quencher most typically used in a Molecular Beacon is Dabcyl. Because the quenching does not involve FRET, there is little, if any, dependence upon donor-acceptor spectral overlap. However, it appears that not all dark quenchers are made equal. In a comprehensive paper by Marras, Kramer and Tyagi,6 the ability of BHQ-1 and BHQ-2 to quench 22 different fluorophores was evaluated. For shorter wavelength fluorophores such as fluorescein, the quenching efficiency was roughly the same as Dabcyl (91% – 93%). However, for dyes emitting in the far red, such as Cy5, the BHQ dyes were far superior – quenching the Cy5 with 96% efficiency, compared to 84% with Dabcyl. This may reflect the BHQ’s ability to form stable, non-fluorescent complexes which can be a plus even in FRET probes. Indeed, recent work suggests that these non-fluorescent complexes will form even in the absence of a hairpin stem structure used by Molecular Beacons.7
"Black Hole Quencher", "BHQ-0", "BHQ-1", "BHQ-2" and "BHQ-3" are trademarks of Biosearch Technologies, Inc., Novato, CA. The BHQ dye technology is the subject of pending patents and is licensed and sold under agreement with Biosearch Technologies, Inc.. Products incorporating the BHQ dye moiety are sold exclusively for R&D use by the end-user. They may not be used for clinical or diagnostic purposes and they may not be re-sold, distributed or re-packaged.







References
- T. Forster, Ann Phys. (Leipzig), 1948, 2, 55-75.
- A.T. Yeung, B.P. Holloway, P.S. Adams, and G.L. Shipley, Biotechniques, 2004, 36, 266-70, 272, 274-5.
- C.T. Chen, H. Wagner, and W.C. Still, Science, 1998, 279, 851-3.
- E.A. Lukhtanov, M. Metcalf, and M.W. Reed, American Biotechnology Laboratory, 2001, September.
- S. Tyagi and F.R. Kramer, Nature Biotechnology, 1996, 14, 303-308.
- S.A.E. Marras, F.R. Kramer, and S. Tyagi, Nucleic Acids Res., 2002, 30, E122.
- M.K. Johansson, H. Fidder, D. Dick, and R.M. Cook, J Am Chem Soc, 2002, 124, 6950-6956.
Product Information
- Glen Report 17.11: Introduction
- Glen Report 17.12: Products for siRNA Research
- Glen Report 17.13: UniCap Phosphoramidite, AN alternative to acetic anhydride capping
- Glen Report 17.14: Expanding Our Repertoire of Dark Quenchers: Black Hole Quenchers
- Glen Report 17.15: 2'-Fluoro-RNA Monomers
- Glen Report 17.16: Internally Quenched Nucleotide Fluorescent Reporters
- Glen Report 17.17: AB 3900 and MerMade Columns

