The RNA analogue, 2’-deoxy-2’-fluoro-RNA (2’-F-RNA), has always been intriguing to us and we have offered the C and U analogues for some time. Unfortunately, the A and G analogues have not been available commercially due to their extremely complex chemical syntheses.1 We are happy to report that all four analogues are now available following production by a novel and elegant enzymatic transformation.



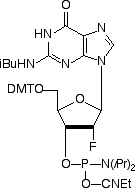
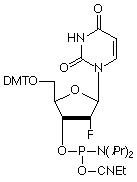
Fluorine has an interesting combination of properties, combining electronegativity similar to a hydroxyl group with size between an oxygen and a hydrogen atom. This combination leads to the ring of a 2’-F-ribonucleoside adopting a C3’-endo conformation and the resulting 2’-F-RNA oligonucleotide adopts an A-form helix on hybridization to a target. Indeed, circular dichroism (CD) spectra of 2’-F-RNA/RNA duplexes indicate that they are A-form and that the sugars have all adopted the C3’-endo pucker.1 An important difference between RNA and 2’-F-RNA is that a hydroxyl group is a hydrogen bond donor while fluorine is a weak acceptor.
In studying antisense oligonucleotides, a group at Isis Pharmaceuticals1 concluded that oligonucleotides hybridized to a target RNA oligonucleotide in the following order of increasing stability: DNA < RNA < 2’-OMe-RNA < 2’-F-RNA. With an RNA target, melting temperature (Tm) was enhanced relative to an antisense DNA oligonucleotide by 1°C per residue for RNA, 1.3 °C for 2’-OMe-RNA, and 1.8 °C for 2’-F-RNA. The stability enhancement for 2’-F-RNA hybridizing to an RNA target was additive for each 2’-F-RNA residue and slightly cooperative – i.e., the DTm per substitution increases as more 2’-F-RNA residues are incorporated into the oligonucleotide. This has led to the use of 2’-F–RNA in aptamers since the resulting aptamers are not only more resistant to nucleases compared to 2’-OH RNA aptamers, but also bind ligands with higher affinities.2 The use, however, of 2’-F-RNA in antisense applications is limited since the 2’-F-RNA exhibits little enhanced nuclease resistance compared to DNA and its hybrid duplex does not activate RNase-H. Interestingly, 2’-F-RNA can be used quite effectively in siRNA applications.
Recent work by Layzer et al., demonstrated that siRNA synthesized with 2’-F pyrimidines showed greatly increased stability in human plasma compared to 2’-OH siRNA.3 They were functional in cell culture and in vivo using BALB/c mice transfected with pGL3 luciferase. Interestingly, though the 2’-F siRNA was significantly more stable than 2’-OH siRNA, they were only slightly more inhibitory over time in cell culture than 2’-OH siRNA; in vivo, their activities were practically the same. The authors note that these results may depend upon the siRNA delivery methodology.
Less has been reported on the stability of duplexes between 2’-F-RNA and DNA. In a study4 of the cleavage of RNA/DNA duplexes by RNase H, 2’-F-Adenosine (2’-F-A) oligonucleotides and chimeras containing 2’-F-A and rA were used to evaluate the ability of the modified RNA strand to promote varying levels of RNase-H activity. The authors measured the Tm of 18-mer oligonucleotides containing rA and/or 2’-F-A to oligo-T18 and found that the homopolymer of 2’-F-A enhanced binding by 0.5° per residue relative to rA. However, chimeras of 2’-F-RNA and rA were unpredictable in their melting behavior and some actually lowered the duplex Tm.
Our own melting experiments of duplexes containing 2’-F-RNA supported these results. We have found that a single substitution of 2’-F-RNA in a mixed base DNA/DNA dodecamer increased the Tm by 1.2 °C. However, further substitutions with two or four 2’-F-RNA residues led to a drop in the Tm by 1.3 °C. Interestingly, a fully substituted 2’-F-RNA/DNA duplex does exhibit higher stability, with the Tm being increased by 0.5° per incorporation.
With a full set of monomers now available, we predict applications for 2’-F-RNA in ribozymes, siRNA and structural DNA research. By making all four monomers available, we hope to open up a full spectrum of research applications for 2’-F-RNA.