Glen Report 32.16: Technical Snippets
How much DBCO is lost if standard iodine oxidation is used?
The answer is it varies. Iodine will cause DBCO to be cleaved from the oligonucleotide, and the rate will be dependent on temperature, iodine concentration and exposure time. For internally located DBCO groups, numerous cycles of iodine exposure can be very problematic. However, even if the DBCO is only added as a 5’ label, the amount of DBCO loss can still be undesirable depending on how the last oxidation step is carried out. To completely avoid this side reaction, we recommend the use of CSO oxidizer for the oxidation of all DBCO-containing oligonucleotides as discussed earlier (https://www.glenresearch.com/reports/gr27-17). Alternatively, we offer DBCO-sulfo-NHS Ester that completely bypasses the exposure of DBCO to oxidization reagents.
Products:
- 5’-DBCO-TEG Phosphoramidite, 10-1941
- DBCO-dT-CE Phosphoramidite, 10-1539
- DBCO-Serinol Phosphoramidite, 10-1998
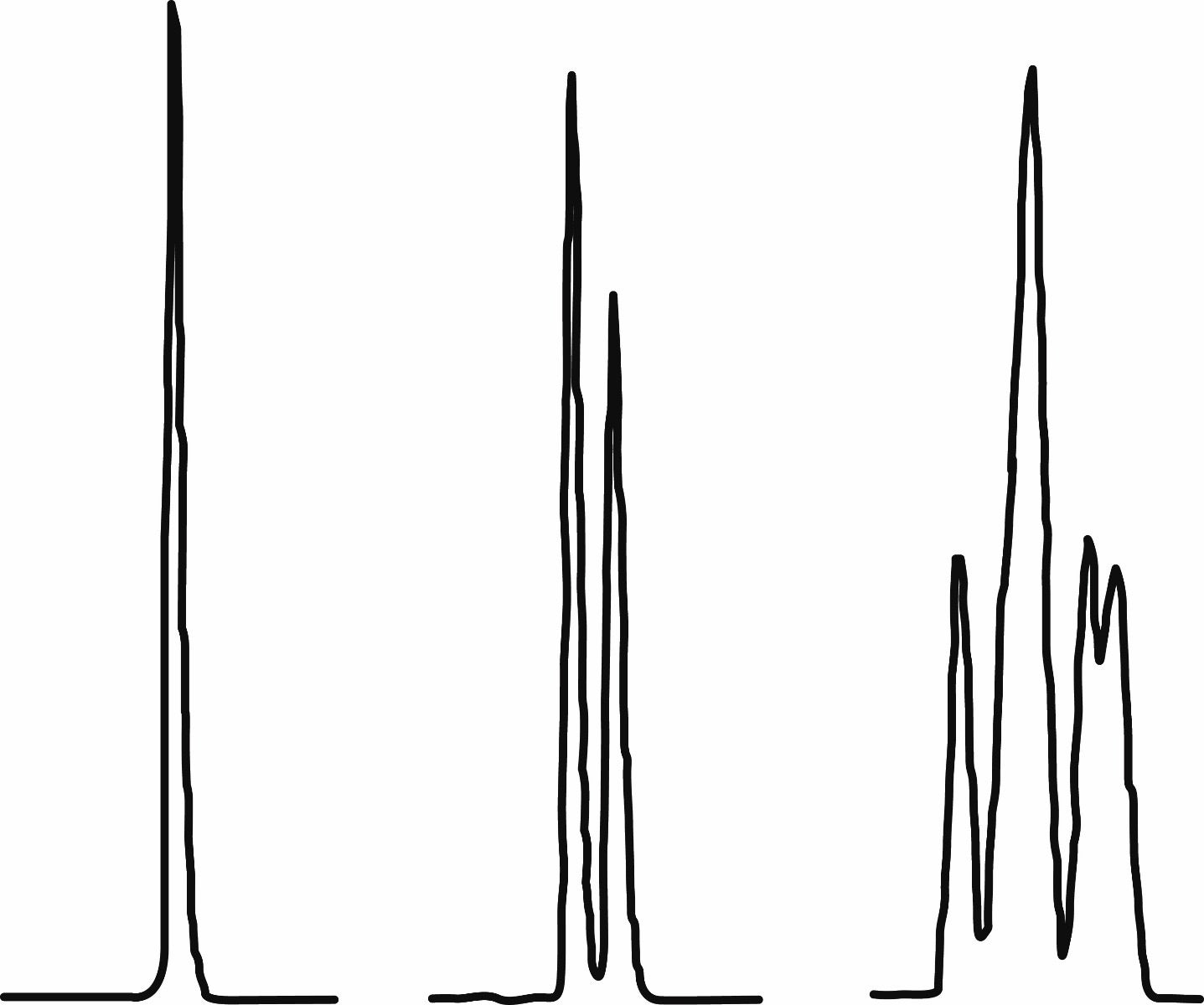
Why is the RP-HPLC chromatogram for phosphoramidites often two peaks and sometimes even more?
|
This is due to the presence of diastereomers. When phosphorus has a lone pair of electrons and three different groups attached to it, as is the case for a phosphoramidite, it is a stereocenter. For a modifier phosphoramidite without other stereocenters, one would typically see only one peak (Figure 1). For a standard DNA phosphoramidite, however, D-2’-deoxyribose causes the phosphoramidite peak to split into two. For an extreme example, our trimer phosphoramidites have as many as eight peaks due to the presence of three phosphorus stereocenters. With all these stereocenters being used to construct oligonucleotides, readers may wonder whether oligonucleotides are a large mix of stereoisomers. Fortunately, that is not the case. After oxidation and deprotection, the phosphorus in the phosphodiesters are no longer stereocenters. Products:
|
|
| A | B | C |
Figure 1. RP-HPLC of three different phosphoramidites. A. 5’-amino-modifier C6; B. dT-CE phosphoramidite; C. TCT trimer phosphoramidite. The relative scale of the x-axis is identical for all three chromatograms.
- Glen Report 32.11: Thiazole Orange as a Fluorogenic Reporter in Oligonucleotide Probes
- Glen Report 32.12: New Product — Thiazole Orange NHS Ester
- Glen Report 32.13: New Product — 2’-Fluoro-Inosine-CE Phosphoramidites
- Glen Report 32.14: New Product — 2'-MOE RNA Phosphoramidites
- Glen Report 32.15: Application Note — Trimer Phosphoramidites
- Glen Report 32.16: Technical Snippets