Glen Report 22.22: New Products – dX and Ferrocene-dT
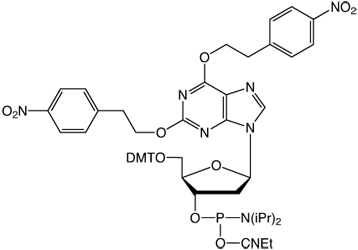
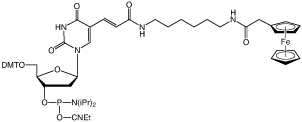
2’-DeoxyXanthosine
2’-DeoxyXanthosine (dX) is a naturally occurring nucleoside that may be derived from oxidative deamination of 2’-deoxyGuanosine (dG). dX has interested researchers for many years because of its potential to base pair with all four natural bases and it has been considered as a potential universal base.1 dX featured in Benner’s attempts to extend the genetic alphabet with a new base pair of dX and pyrimidine-2,4-diamine nucleoside.2 dX has also interested researchers in the field of DNA damage and repair since it is a product of nitric oxide-induced mutagenesis.3
Unfortunately, dX is also a very insoluble nucleoside and it has proved difficult to carry out the chemistry required to make a useful phosphoramidite for incorporating dX into oligonucleotides. However, Benner’s choice of protection for dX with dual nitrophenylethyl (NPE) protecting groups (1) has proved to be the most successful to date.4 This protected dX monomer can be incorporated into oligonucleotides using conventional synthesis. The resulting oligonucleotides are cleaved and deprotected using ammonium hydroxide at room temperature with times dependent on the protecting groups on the regular bases (4 hours for UltraMild monomers, overnight for dmf-dG, and up to 36 hours for iBu-dG). After drying, the NPE protecting groups must be removed using 0.3M tetramethylguanidine/2-nitrobenzaldoxine in water dioxane (1:1) at 70 °C for 48 hours. After evaporation, the product oligonucleotide is desalted using standard techniques.
Ferrocene-dT
With an excellent stability profile, ferrocene has always attracted considerable interest for DNA labelling to generate probes for electrochemical detection.5, 6 A variety of post-synthesis conjugation techniques has been used to attach ferrocene to oligonucleotides, with the most popular technique being the conjugation of amino-modified oligonucleotides with activated ferrocene carboxylates. Such post-synthesis conjugation techniques may be useful but they tend to be inconvenient as well as low yielding. Of course, the problems quickly escalate if several positions need to be conjugated.
We are happy to introduce ferrocene-dT (2) to our product range. Based on our Amino-Modifier C6-dT structure, this product is easily added to oligonucleotides with no disruption of regular hybridization behavior. Multiple incorporations into an oligonucleotide probe are also simply achieved. Oligonucleotides are deprotected using standard techniques. Ferrocene oligonucleotides should be stored under Argon and aqueous solutions should be degassed immediately.
References
- R. Eritja, et al., Nucleic Acids Res, 1986, 14, 8135-53.
- M.J. Lutz, H.A. Held, M. Hottiger, U. Hubscher, and S.A. Benner, Nucleic Acids Res, 1996, 24, 1308-13.
- G.E. Wuenschell, T.R. O’Connor, and J. Termini, Biochemistry, 2003, 42, 3608-16.
- S.C. Jurczyk, J. Horlacher, K.G. Devined, S.A. Benner, and T.R. Battersby, Helv Chim Acta, 2000, 83, 1517-1524.
- A.E. Navarro, et al., Nucleic Acid Res, 2004, 32, 5310-5319.
- H. Brisset, A.E. Navarro, N. Spinelli, C. Chaix, and B. Mandrand, Biotechnology Journal, 2006, 1, 95-98.
Product Information
dX-CE Phosphoramidite (10-1537)
Ferrocene-dT-CE Phosphoramidite (10-1576)
- Glen Report 22.21: 2’-F-Arabinonucleic Acid (2’-F-ANA)
- Glen Report 22.22: New Products – dX and Ferrocene-dT
- Glen Report 22.23:; New Product: 0.5M CSO for non-aqueous oxidation in DNA synthesis
- Glen Report 22.24: New Product - Universal Tosyl Phosphoramidite
- Glen Report 22.25: New Products - Glen Gel-Pak™ DNA/RNA Desalting and 1000Å Glen UnySupport™ Frits
- Glen Report 22.26: New Products – Azide Tags for Click Chemistry
- Glen Report 22.27: Deprotection – Volume 5 – On-Column Deprotection of Oligonucleotides in Organic Solvents
- Glen Report 22.28: Technical Brief - Symmetrically Branched Four-Arm DNA
- Glen Report 22.29: Technical Brief - Crosslinking with Click Chemistry
- Glen Report 22.210: Glen-Pak™ Product Update: New Protocols for Purification

