Glen Report 19.28: Versatile New Reagents – Pyrene-dU and Perylene-dU
Pyrene has intrigued researchers ever since Förster and Kasper described its ability to form 'excited state dimers', later to be known as excimers.1 They observed that while dilute solutions of pyrene fluoresce violet, at higher concentrations the fluorescence shifts 100 nm and becomes blue. This unstructured, long-wavelength emission arises from the formation of a charge-transfer complex between a pyrene in the excited state and another pyrene in the ground state. Since this complex must form within the lifetime of the excited state, the two pyrene molecules must be in close proximity - an ideal situation for developing DNA probes. By having two pyrene-labeled DNA probes, one with the pyrene on the 5’ terminus and the other on the 3’, the two pyrenes could be brought together by hybridizing in tandem to the target sequence, leading to excimer formation.2 This technique has been used recently to probe mRNA in cells, taking advantage of the relatively long fluorescence life time of the pyrene excimer.3
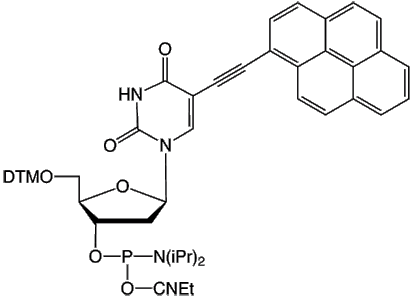
An interesting development came when Korshun attached the pyrene to the 5 position of deoxyuridine through a triple bond (Figure 1).4 By doing so, the pyrene is electronically coupled to the deoxyuridine base as shown by the redshifting of the pyrene absorbance by more than 50 nm compared to the unsubstituted 1-ethynylpyrene.5 This electronic coupling of the base and the pyrene makes the fluorescence of the pyrene sensitive to the base pairing of the dU portion of the molecule, allowing the discrimination between perfect and one base mismatched targets.6 This coupling also allows photoinduced charge transfer to occur upon excitation of the pyrene, essentially ‘injecting’ electrons into the duplex.7 The only downside is a substantial reduction of the Stokes’ shift of the excimer’s fluorescence compared to the monomer, dropping from circa 100 to 14 nm, as shown in Figure 2A on the Back Page.
| λ (abs) | λ (emission) | λ (emission excimer) | |
|---|---|---|---|
| Pyrene-dU | 402 nm | 472 nm | 486 nm |
| Perylene-dU | 473 nm | 490 nm | Not Determined |
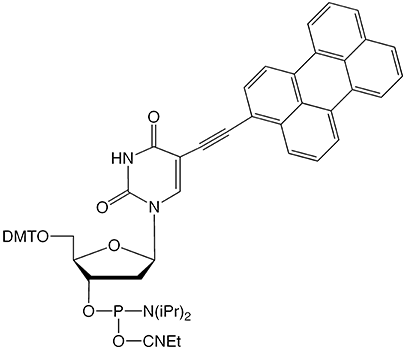
To complement the Pyrene-dU, we are also introducing its longer wavelength cousin, Perylene-dU (Figure 1). The Perylene-dU is another fluorescent polycyclic aromatic hydrocarbon that can form excimers. As with the Pyrene-dU, the Perylene analog has been shown to be sensitive to its hybridization state.8 When used in tandem, perylene and pyrene have been shown to form exciplexes9 giving rise to a broad range of fluorescence emission depending upon the sequence and spacing between the fluorophores.
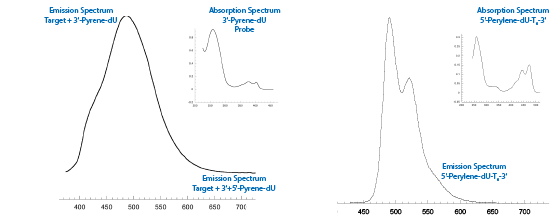
Absorption and emisssion data for Pyrene-dU and Perylene-dU are collected in the Table and the emission spectrum for Perylene-dU with the absorption spectrum as an insert is shown in Figure 2B on the Back Page.
| FIGURE 2a: Fluorescence SpectrUM of pyrene-dU with Absorbance Spectrum insertCAPtiO 2 HERE | FIGURE 2b: Fluorescence SpectrUM of PERYLENE-dU with Absorbance Spectrum insert |
 |
|
| Figure 2A: Emission of 5’-AGC ATG AAT CAT CGA CTC AAG GT-Pyrene-dU-3’ when annealed to the target sequence 5’-GTT AGT CGT ACG TAC GCT AGA TCC GAA ACC TTG AGT CGA TGA TTC ATG CT-3’. Upon addition of 5'-Pyrene-dU-CG GAT CTA GCG TAC GTA CGA CTA AC-3’, there is a reduction in the pyrene emission at 414 nm and a redshift of the emission to 486 nm, indicating the formation of the excimer. Inset is the 3’-Pyrene-dU probe absorbance spectrum. Figure 2B: Emission spectrum of 5'-Perylene-dU-T6-3'. Inset is the 5'-Perylene-dU-T6-3' absorption spectrum. | |
We are happy to introduce these interesting new products to our repertoire of unusual bases.
References
- T. Förster, Kasper, K., Z. Elektrochem., 1955, 59, 976.
- P.L. Paris, J.M. Langenhan, and E.T. Kool, Nucleic Acids Res., 1998, 26, 3789-93.
- A.A. Marti, et al., Nucleic Acids Res, 2006, 34, 3161-8.
- V.A. Korshun, et al., Bioorg Khim, 1996, 22, 923-925.
- A. Tifonov. Technische Universität München, 2005.
- G.T. Hwang, Y.J. Seo, S.J. Kim, and B.H. Kim, Tetrahedron Lett, 2004, 45, 3543-3546.
- S.T. Gaballah, G. Collier, and T.L. Netzel, J Phys Chem B, 2005, 109, 12175-81.
- M.V. Skorobogatyi, et al., Chembiochem, 2006, 7, 810-6.
- J.N. Wilson, J. Gao, and E.T. Kool, Tetrahedron, 2007, 63, 3427-3433.
Product Information
Pyrene-dU-CE Phosphoramidite (10-1590)
Perylene-dU-CE Phosphoramidite (10-1591)
- Glen Report 19.21: Glen-Pak™ Cartridges – The Ultimate Purification Cartridges
- Glen Report 19.22: Technical Brief - Procedure for the Synthesis and Deprotection of Synthetic RNA
- Glen Report 19.23: Improved RNA Synthesis Using the Q-Linker for Faster Product Cleavage from Solid-Phase Supports
- Glen Report 19.24: New Lines of Monomers - HT for High Throughput and LC for Low Cost RNA
- Glen Report 19.25: AP-dC is compatible with PCR and is Highly Fluorescent
- Glen Report 19.26: Pyrrolidine CE Phosphoramidite: A New Base Excision Repair Inhibitor
- Glen Report 19.27: New Products - 2,6-Diaminopurine-TOM and Fmoc-Amino-Modifier C6 dT Amidites
- Glen Report 19.28: Versatile New Reagents – Pyrene-dU and Perylene-dU
- Glen Report 19.29: Technical Brief - About Activators: Now and Tomorrow
- Glen Report 19.210: Introducing Thiophosphoramidites

