Glen Report 19.26: Pyrrolidine CE Phosphoramidite: A New Base Excision Repair Inhibitor
DNA is constantly under attack. Alkylating agents, ionizing radiation and oxidative stress can induce base modification or strand scission that, left unchecked, can lead to the development of cancer. Thankfully, our cells are equipped with DNA damage-monitoring and repair enzymes to correct the damage via base excision repair (BER) or nucleotide excision repair (NER). Ironically, though, the up-regulation of these repair enzymes in cancerous cells frequently causes the development of drug resistance against chemotherapeutic reagents.
One of the most studied repair mechanisms is probably the base excision DNA repair pathway. In this pathway, DNA glycosylases recognize the damaged bases and catalyze their excision through hydrolysis of the N-glycosidic bond. Attempts to understand the structural basis for DNA damage recognition by DNA glycosylases have been hampered by the short-lived association of these enzymes with their DNA substrates. To overcome this problem, the design and synthesis of inhibitors that form stable complexes with DNA glycosylases are essential. Complexes can then be studied biochemically and structurally.
Toward this end, the Verdine group at Harvard synthesized a pyrrolidine analog that mimics the charged transition state of the enzyme-substrate complex, as shown in Figure 1. When incorporated into double-stranded DNA, they found the pyrrolidine analog (PYR), introduced as the phosphoramidite (1), forms an extremely stable complex with the DNA glycosylase AlkA, exhibiting a dissociation constant in the pM range and potently inhibited the reaction catalyzed by the enzyme.1
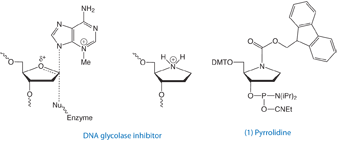
Later, the same group in collaboration with international researchers investigated the interaction of this inhibitor with a variety of additional DNA glycosylases. With the exception of uracil DNA glycosylase, all the glycosylases tested bind specifically to PYR-containing oligonucleotides2 – providing an elegant means by which to study a broad range of DNA glycosylases and BER proteins. (Of course, we would be remiss not to mention Vern Schramm when discussing enzymatic transition state analogs and would recommend his excellent review of the subject.3) One might be tempted to think that any abasic analog, such as our dSpacer, (abasic furan – 10-1914), might also bind to AlkA. However, the charge is clearly necessary as shown by Verdine’s work.1 When the dSpacer was incorporated rather than the pyrrolidine, the Kd was estimated to be 10,000-fold lower.
In any event, it is important to note that pyrrolidine is not directly comparable to our existing abasic phosphoramidite (dR precusor - 10-1924) or dSpacer as the new product will introduce a charge residue. Since the paper that Takeshita et al. published in 19874, the dSpacer/abasic furan (which was introduced a few years later) has been extensively used to study, for example, the fidelity of polymerases when the enzyme encounters an abasic site. The paper published by Hogg et al. in 2006 and several citations in this paper5 are good examples of such research. Other information and comparison of various building blocks for the incorporation of abasic lesions can be found in a paper written by Shigenori Iwai.6
Given the importance of analogues involved in base lesions and their repair hold for Glen Research, one might wonder why we haven’t made the Pyrrolidine CE Phosphoramidite ((1) in Figure 1) available before now. It was difficult to provide this analogue because the chemistry used by the Verdine group1 was complex and not really well suited for production purposes. With the development of an alternative synthetic route, we are happy to provide this new product to the research community studying DNA repair.
For the chemists in charge of oligo synthesis, the coupling time of the PYR phosphoramidite should ideally be extended to 5 minutes (tested with 1-H Tetrazole as activator) and the deprotection procedure is standard. We have also carried out accelerated stability studies to confirm that that the PYR phosphoramidite is stable even though it contains an Fmoc protecting group. Fortunately, the stability of the PYR phosphoramidite was found to be more than acceptable.
We hope that this addition to our catalog will be helpful to researchers in the field of DNA base excision repair.
References
- O.D. Scharer, et al., J. Am. Chem. Soc., 1995, 117, 6623-6624.
- O.D. Scharer, et al., J. Biol. Chem., 1998, 273, 8592-8597.
- V.L. Schramm, Arch Biochem Biophys, 2005, 433, 13-26.
- M. Takeshita, et al., J. Biol. Chem., 1987, 262, 10171-10179.
- M. Hogg, W. Cooper, L. Reha-Krantz, and S.S. Wallace, Nucleic Acids Res., 2006, 34, 2528-2535.
- S. Iwai, Nucleosides Nucleotides & Nucleic Acids, 2006, 25, 561.
Product Information
- Glen Report 19.21: Glen-Pak™ Cartridges – The Ultimate Purification Cartridges
- Glen Report 19.22: Technical Brief - Procedure for the Synthesis and Deprotection of Synthetic RNA
- Glen Report 19.23: Improved RNA Synthesis Using the Q-Linker for Faster Product Cleavage from Solid-Phase Supports
- Glen Report 19.24: New Lines of Monomers - HT for High Throughput and LC for Low Cost RNA
- Glen Report 19.25: AP-dC is compatible with PCR and is Highly Fluorescent
- Glen Report 19.26: Pyrrolidine CE Phosphoramidite: A New Base Excision Repair Inhibitor
- Glen Report 19.27: New Products - 2,6-Diaminopurine-TOM and Fmoc-Amino-Modifier C6 dT Amidites
- Glen Report 19.28: Versatile New Reagents – Pyrene-dU and Perylene-dU
- Glen Report 19.29: Technical Brief - About Activators: Now and Tomorrow
- Glen Report 19.210: Introducing Thiophosphoramidites

