Glen Report 7.14: UltraMild DNA Synthesis
In recent years, the synthesis of labelled oligonucleotides has become virtually a standard procedure in many labs. Many labelling reagents, e.g., biotin, fluorescein are now available as ß-cyanoethyl (CE) phosphoramidites and this provides a rapid means of producing the appropriate oligonucleotides directly. Prior to the availability of these labelling reagents as CE phosphoramidites, conjugations were carried out using the solution phase reaction of amino-or thiol- modified oligonucleotides with the appropriately functionalized label. Labels which are currently available as CE phosphoramidites have one property in common - they must be stable to strongly alkaline conditions required for removal of the base protecting groups. This property is lacking in several interesting dyes and labels and so we have been seeking an alternative protecting scheme for the normal CE phosphoramidites which allows UltraMILD deprotection and should not react with a wider variety of tags and labels
Expedite chemistry, as offered by Biosearch, does allow for much milder deprotection (ammonium hydroxide/2h/RT) but even this is too harsh for some prospective CE phosphoramidite labels. Our goal, therefore, was to find a synthesis system employing monomer protecting groups which can be removed without the use of harsh basic reaction conditions
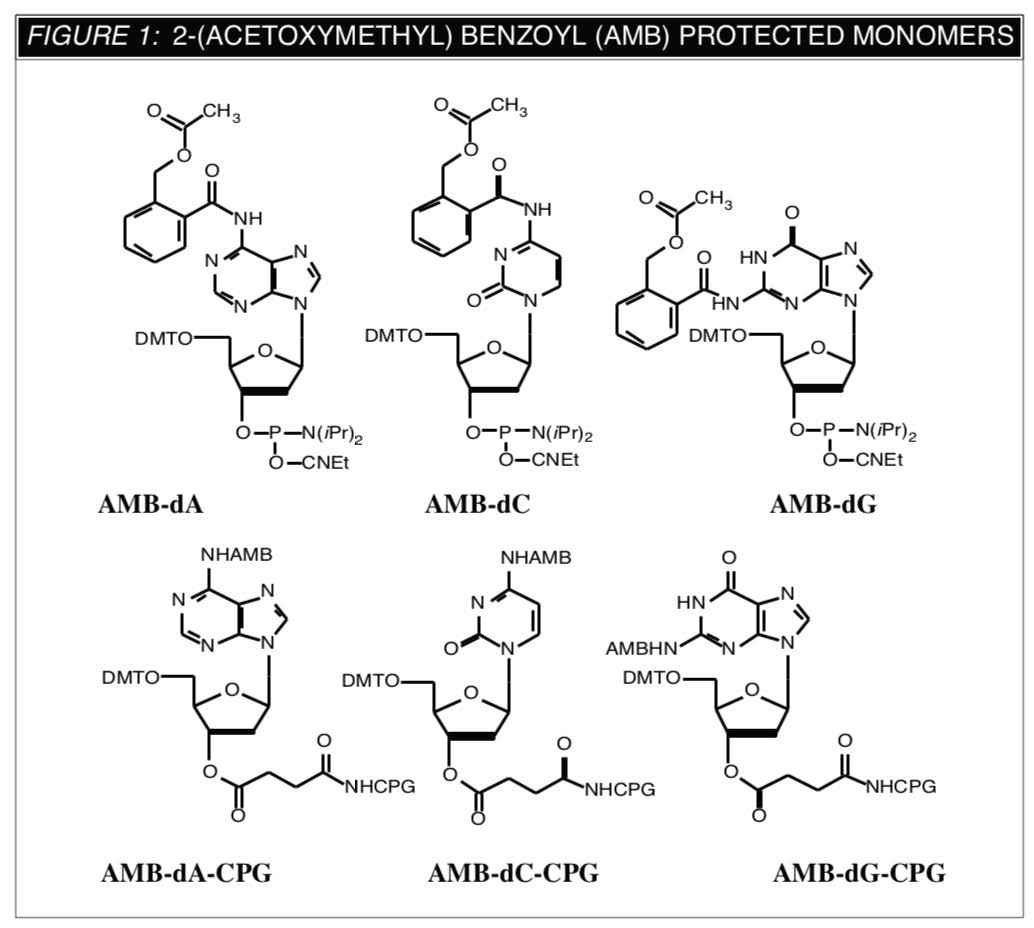
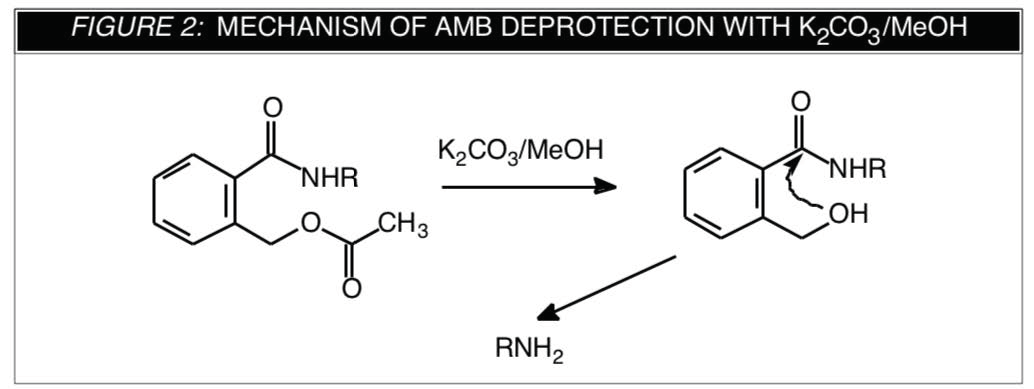
We were prompted1 to look at work which had been carried out to prepare base-labile backbones, e.g., methyl phosphotriesters2 and methyl phosphonates3. As described by Dutch researchers2,3, the 2-(acetoxy- methyl)benzoyl (AMB) group is used for base protection and later removed using anhydrous potassium carbonate in methanol (90 minutes/RT). Structures of the AMB protected monomers and supports are shown in Figure 1 and a suggested mechanism for their removal is shown in Figure 2.
References
(1) L.J. Marnett, Vanderbilt University Medical Center, Personal Communication.
(2) W.H.A. Kuijpers, J. Huskens and C.A.A. Van Boeckel, Tetrahedron Lett., 1990, 31, 6729-6732.
(3) W.H.A. Kuijpers, E. Kuyl-Yeheskiely, J.H. Van Boom, and C.A.A. Van Boeckel, Nucleic Acids Res., 1993, 21, 3493-3500.
AMB products have been discontinued. Please see:
UltraMild Base Protection Phosphoramidites and Supports

