Glen Report 7.11: Propynl-2'-OMe-RNA, Br-U, I-U
- ANTISENSE PROBES, CROSSLINKING
Researchers have continued to explore the potential uses of C-5 propynyl pyrimidine derivatives of oligonucleotides. Caltech investigators1 have examined the effect of C-5 propynyl-dU on triple helix formation. Groups from Gilead Sciences and The Agouron Institute2 have measured the specific effect of a series of C-5 propyne oligonucleotides on HIV mRNA targets
Froehler and coworkers3 have already examined the effect of C-5 propynyl pyrimidine modifications in the behavior of 2'-O-allyl-RNA. Since our main focus in RNA monomer supply is on 2'-OMe-RNA, we believe that the enhanced binding of C-5 propynyl groups in 2'-OMe-RNA would be beneficial in the preparation and use of antisense RNA probes. We, therefore, have prepared C5-propynyl-2'-OMe-C and C5-propynyl-2'-OMe-U-CE Phosphoramidites. Structures and Ordering Information are shown on the Back Page. It should be cautioned that the U analogue is quite insoluble in acetonitrile and we recommend the use of THF as solvent and/or manual coupling for this monomer.
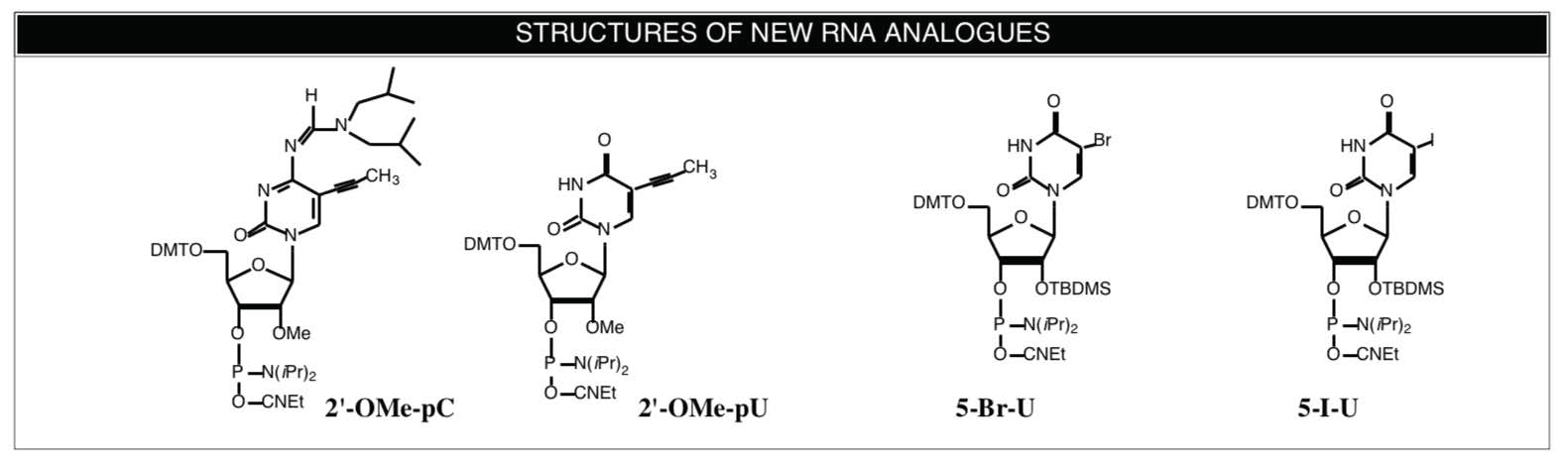
Br-U, I-U-CE Phosphoramidites
Interest in crosslinking experiments has not been restricted to DNA protein interactions and we have been asked to provide halogenated RNA monomers. The two Uridine derivatives 5-Br-U and 5-I-U4 are now available from Glen Research.
References
(1) N. Colocci and P.B. Dervan, J. Amer. Chem. Soc., 1994, 116, 785-786.
(2) S.D. Fenster, R.W. Wagner, B.C. Froehler, and D.J. Chin, Biochemistry, 1994, 33, 8391-8398.
(3) B.C. Froehler, R.J. Jones, X.D. Cao, and T.J. Terhorst, Tetrahedron Lett., 1993, 34, 1003-1006.
(4) K. Shah, H. Wu, and T.M. Rana, Bioconjugate Chemistry, 1994, 5, 508-512.
Product Information
The C5-propynyl-2'-OMe-CE Phosphoramidites have been discontinued.
Br-U-CE Phosphoramidite (10-3090)
5-I-U-CE Phosphoramidite (10-3091)

