Glen Report 23.16: Technical Brief - Labelling Carboxy-Modifiers with Multiple Reporter Molecules
Conjugating reporter molecules to DNA has been an important functionalizing technique for use in many diagnostic, therapeutic and research applications. One of the most popular conjugation methods is the reaction of an amino group with an activated carboxylic acid to form an amide linkage.
N-hydroxysuccinimyl (NHS) Esters are activated carboxylic acids that react preferentially with primary amines and can be used in aqueous and organic solvents. For the conjugation of oligonucleotides with NHS Esters, the most common approach is to include a primary amine in the oligonucleotide sequence using an amino-modifier attached to a nucleoside, or to one of the termini, or to an internal non-nucleosidic linker. After the synthesis is complete, the oligo is deprotected and cleaved from the support. The amine-labelled oligo is then reacted with an NHS Ester to form the oligonucleotide conjugate. An excess of the NHS Ester is required to complete the reaction in a short amount of time and the excess NHS Ester as well as hydrolysis by-products are removed by desalting or HPLC purification.
Several years, ago we introduced Carboxy-Modifier C10 (10-1935) (1) and later NHS-Carboxy-dT (10-1535) (2) which use an alternative strategy for labelling oligonucleotides. These products place the NHS Ester in the protected oligo on its solid support instead of on the label. A major benefit of this approach is to allow the incorporation of dyes and other reporter molecules that are not available as phosphoramidites. This approach also greatly simplifies purification as the excess reagents are conveniently washed away while the oligo is still on the support. There is also the advantage that excess reporter molecule can be recovered from the reaction solution. In this article, we focus primarily on labelling within the sequence using NHS-Carboxy-dT.
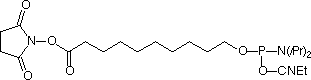
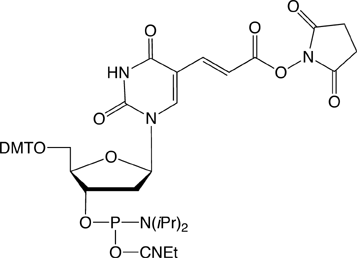
Earlier, we described a simple protocol where the NHS-Carboxy-dT was incorporated using conventional phosphoramidite chemistry, the synthesis was paused to conjugate the label, and then the synthesis completed. A caveat in this protocol (and Scheme 1) is that the label needs to be compatible with one of the methods for cleaving and deprotecting oligos and must not support branching.
We originally investigated the possibility of synthesizing an oligo that includes one or several NHS-Carboxy-dT residues to completion and then performing the conjugation. Our initial results were acceptable but marginal due to considerable hydrolysis of the NHS Ester residues prior to conjugation. However, we have since discovered that the choice of oxidizer makes a profound difference in the stability of the NHS Ester during synthesis. When we revisited the synthesis conditions, we found that two oxidizers are quite effective for use with NHS-Carboxy-dT with no undesired hydrolysis of the ester - 0.02M Iodine in THF/Water/Pyridine (40-4330 or 40-4132) and 0.5M CSO in Acetonitrile (40-4632).
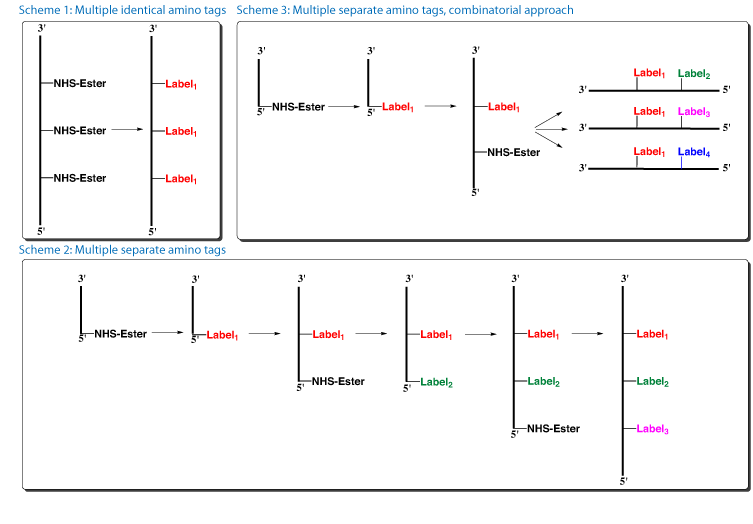
With our finding that NHS-Carboxy-dT is stable to repetitive oxidative cycles, several new options become available for oligos containing multiple modifications.
- One or several NHS-Carboxy-dT residues can now be labelled efficiently with the same amino tag on the support after the oligo synthesis, with no need to pause the synthesis for each conjugation (Figure 2, Scheme 1).
- Several NHS-Carboxy-dT residues can be labelled with separate amino tags by pausing the synthesis and carrying out each conjugation reaction separately (Figure 2, Scheme 2).
- NHS-carboxy-dT could be used in a combinatorial approach for synthesizing families of labelled oligos. In this case, a single oligo synthesis could yield many oligos with different labels (Figure 2, Scheme 3).
- The procedure is compatible with automated protocols.
To illustrate these processes, we synthesized oligos that had two or three incorporations of NHS-Carboxy-dT.
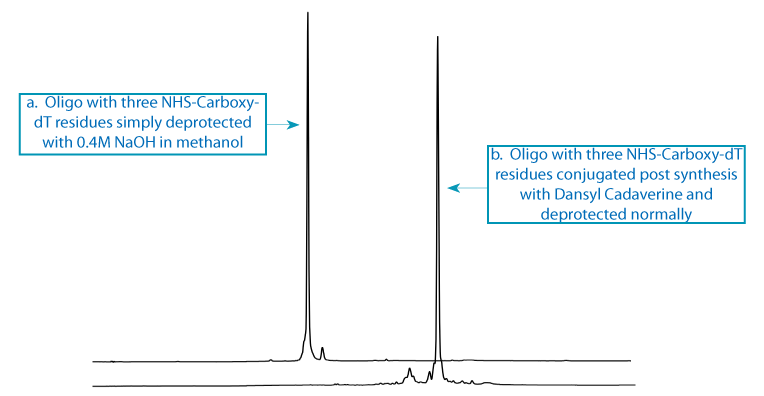
Scheme 1: The first oligo support with three incorporations of NHS-Carboxy-dT was split into two portions. One aliquot, the control, was treated with 0.4M NaOH in methanol to form carboxy-dT (Figure 3a). The second aliquot was conjugated with Dansyl Cadaverine to form the triply dansyl labelled oligo once conventional deprotection was completed (Figure 3b).
Scheme 2: For the oligo with two incorporations of NHS-Carboxy-dT, the synthesis was paused to label the first NHS-Carboxy-dT residue with Dansyl Cadaverine. The excess reagents were washed away and the synthesis completed, including the second incorporation of NHS-Carboxy-dT. After synthesis but prior to deprotection or cleavage, the oligo was labelled with Dabsylamine to form the Dansyl-Dabsyl labelled oligo. The results are compelling demonstrating that the oligo was virtually quantitatively double labelled with Dansyl Cadaverine and Dabsylamine (Figure 4).

We conclude that on-column labelling of oligonucleotides containing a carboxylate NHS ester is a highly efficient procedure that is a preferred alternative to post synthesis labelling in aqueous solution. Not every reporter molecule can be added using this technique since the conjugated oligonucleotide still has to be deprotected and the labels must survive this treatment. However, in conjunction with UltraMild DNA synthesis and suitable deprotection strategies, NHS-Carboxy phosphoramidite labelling offers a simple and direct approach to synthesizing oligo conjugates that are not compatible with standard deprotection conditions or unavailable as phosphoramidites.
Procedures
Different solvents have been used including DMSO, Acetonitrile, and Dichloromethane. The choice of solvent depends on the properties of the label.
On-Column Labelling Reaction (1.0 µmole scale)
- Dissolve amino-modified label (5-10 equivalents) in 1 mL anhydrous solvent.
- Add 0.5 µL of diisopropylethylamine (DIEA) to the solution.
- Using two syringes to attach to the column, conjugate label solution for 2 hours at RT.
- Rinse with 2 x 1 mL each of DMSO and acetonitrile.
- Complete synthesis DMT-Off or DMT-ON.
- Purify by desalting or DMT-ON purification procedure.
Post-synthesis On-Column Labelling
- Transfer 0.2 µmole of support to a clean dry vial.
- Dissolve amino-modified label (5-10 equivalents) in 1 mL anhydrous solvent.
- Add 0.5 µL of DIEA to the solution.
- Transfer 1 mL of amine solution to support.
- Heat in water bath for four hours at 37 °C, agitating every hour.
- Decant and wash with 1 mL each of DMSO, 10% diethylamine (DEA) in acetonitrile, methanol, and acetonitrile.
- Cleave and deprotect using a method compatible with label.
- Purify by desalting or DMT-ON purification procedure.
Product Information
- Glen Report 23.11: DNA Methylation Revisited
- Glen Report 23.12: New Products Prevent Branching at Secondary Amines using DCI Activator
- Glen Report 23.13: New Products – Click Chemistry Update
- Glen Report 23.14: New Product – Ultrafast Photo Cross-Linker
- Glen Report 23.15: Product Update - Unnatural Base Pairs
- Glen Report 23.16: Technical Brief - Labelling Carboxy-Modifiers with Multiple Reporter Molecules
- Glen Report 23.17: Technical Brief - Chemical Phosphorylation – Considering the Options

