Glen Report 23.14: New Product – Ultrafast Photo Cross-Linker
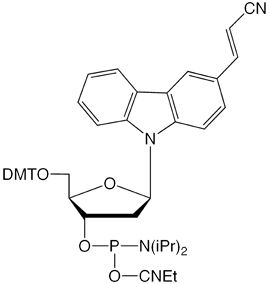
(1) CNVK-CE-Phosphoramidite
The nucleoside analogue, CNVK, can be readily converted into a CE phosphoramidite (1) and its use in oligonucleotide synthesis is straightforward. The modified oligo can also be simply deprotected by a variety of popular techniques.
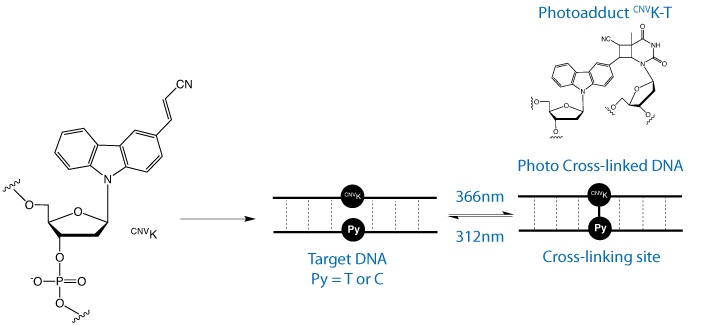
As shown in Figure 2, irradiation of a duplex containing a single incorporation of CNVK at 366nm led to 100% cross-linking to thymine base in 1 second, although complete cross-linking to cytosine takes 25 seconds.1 A 30 second irradiation time should cover all situations. In addition, it was demonstrated that the purine bases were unreactive to cross-linking, allowing differentiation between pyrimidines and purines at the target site. The authors also determined the effect of sequence contexts around the CNVK site and demonstrated that the identity of bases on either side of the cross-linking site has little effect on the reaction. Once cross-linked, the UV melting temperature of the duplex was raised by around 30 °C relative to the duplex before irradiation.
In an accompanying review on our web site (Crosslink.pdf) of cross-linking strategies over the last 15 years, the potential of 3-cyanovinylcarbazole nucleoside (CNVK) for photo-induced cross-linking was clearly apparent.1 When CNVK is incorporated into an oligonucleotide, very rapid cross-linking to the complementary strand can be induced at one wavelength and rapid reversal of the cross-link is possible at a second wavelength. Neither wavelength has the potential to cause significant DNA damage.
Complete reversal of the cross-link takes place at 312nm in 3 minutes. This facile reversal reaction is, therefore, accomplished with no damage to normal DNA.
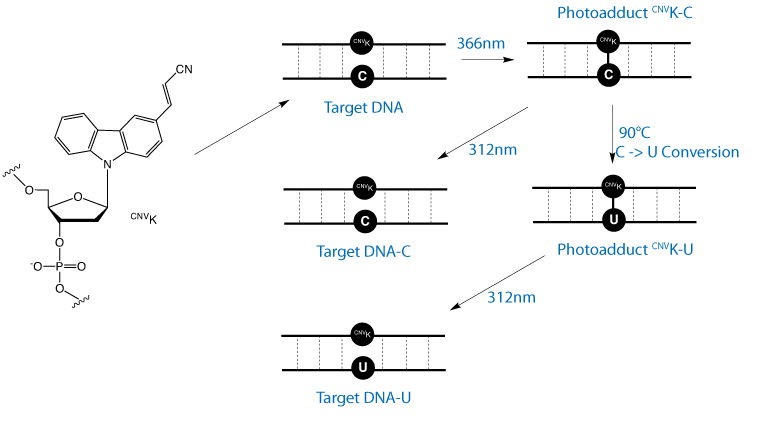
In a later publication, as shown in Figure 3, a further application of this cross-linking technique was investigated.2When CNVK was cross-linked with a dC residue in duplex DNA, heating at 90°C for 3.5 hours led to deamination of the cytosine base to form uracil in the complementary strand. Reversal of the cross-link at 312nm led to a DNA strand in which dC had been converted to dU. The authors showed that this transformation is specific for the dC residue opposite the CNVK and any further adjacent dC residues are unaffected. Similarly, the authors have shown that CNVK can be cross-linked to an adjacent RNA strand.3
We are happy to introduce CNVK-CE phosphoramidite. We expect applications for CNVK in research in general interstrand cross-linking studies, gene expression, and DNA and RNA mutation.
References
- Y. Yoshimura, and K. Fujimoto, Org Lett, 2008, 10, 3227-30.
- K. Fujimoto, K. Konishi-Hiratsuka, T. Sakamoto, and Y. Yoshimura, ChemBioChem, 2010, 11, 1661-4.
- Y. Yoshimura, T. Ohtake, H. Okada, and K. Fujimoto, ChemBioChem, 2009, 10, 1473-6.
Product Information
- Glen Report 23.11: DNA Methylation Revisited
- Glen Report 23.12: New Products Prevent Branching at Secondary Amines using DCI Activator
- Glen Report 23.13: New Products – Click Chemistry Update
- Glen Report 23.14: New Product – Ultrafast Photo Cross-Linker
- Glen Report 23.15: Product Update - Unnatural Base Pairs
- Glen Report 23.16: Technical Brief - Labelling Carboxy-Modifiers with Multiple Reporter Molecules
- Glen Report 23.17: Technical Brief - Chemical Phosphorylation – Considering the Options

