Glen Report 23.15: Product Update - Unnatural Base Pairs
For the last several years, Glen Research has collaborated with TagCyx Biotechnologies on the commercial development of an unnatural base pair that relies on hydrophobic interaction rather than the normal hydrogen bonding of the standard A – T and C – G base pairs. In this article, we will briefly outline the strategies for using the existing product line. We will also introduce a new highly fluorescent nucleoside analogue and suggest some applications for its use in this unnatural base pair environment.
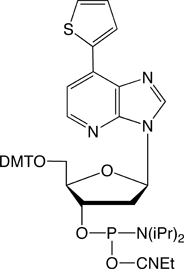
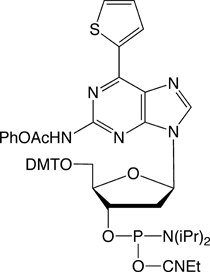
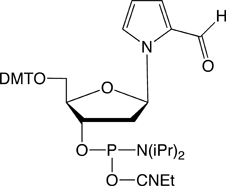
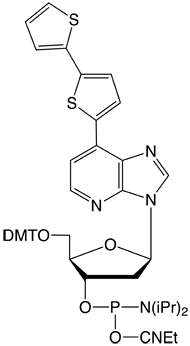
Figure 1 shows all of the products that are currently commercially available for this technology, including dDss, the new fluorescent analogue. Although the array of products may be complicated at first glance, the set breaks down simply as they appear in the TagCyx name as y and x:
| y | x |
|---|---|
| DNA | |
| dDs | dPa |
| dDss | dPa |
| ds | dPa |
| RNA | |
| sTP | BiotinPaTP |

Potential applications make use of the varying physical properties of these nucleoside analogues.
DNA Research
dDs is a weakly fluorescent and stable nucleoside analogue that can be readily incorporated into oligonucleotides for studies where dPa is the complementary “base”, as shown in Figure 2.1

ds is a fluorescent analogue which also pairs with dPa. Pac-ds CE Phosphoramidite has a phenoxyacetyl (Pac) protecting group so is best used with Pac anhydride as the capping reagent. The fluorescence of ds is very sensitive to its environment and this attribute makes ds very useful in structural analysis.2
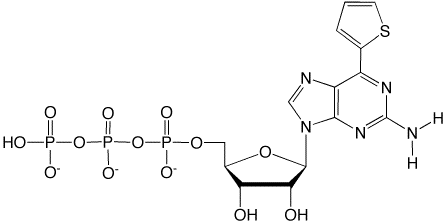
As shown in Figure 3, dDss is strongly fluorescent and is useful as a fluorescent tag for DNA detection. dDss also forms a base pair with dPa.3

RNA Research
Using template DNA containing dDs, RNA transcription using BiotinPaTP leads to RNA biotinylated at sites complementary to dDs. The presence of biotin can be used to capture and immobilize the transcribed RNA.4
Similarly, a DNA template containing dPa can be used along with sTP to generate transcribed RNA containing s at defined locations. Since the fluorescence of s is sensitive to its environment, as illustrated in Figure 4, partial structural analysis of the transcribed RNA can again be developed.5

Properties of dDss
dDss-CE Phosphoramidite is incorporated into oligonucleotides by standard procedures. The modified oligonucleotides can be deprotected by any of the popular procedures.3 dDss has an absorption maximum at 380nm with an extinction coefficient of 31,000 L/mole. With an emission maximum for dDss at 470nm, dabcyl would be a good quencher.
The future for this unnatural base pair family is promising and we expect to see further refinements in the base pairs for specific applications in the future.6
References
- I. Hirao, et al., Nucleic Acids Symp Ser (Oxf), 2006, 33-4.
- T. Mitsui, M.Kimoto, R.Kawai, S.Yokoyama, and I. Hirao, Tetrahedron, 2007, 35, 3528-3537.
- M. Kimoto, T. Mitsui, S. Yokoyama, and I. Hirao, J Am Chem Soc, 2010, 132, 4988-4988.
- K. Moriyama, M. Kimoto, T. Mitsui, S. Yokoyama, and I. Hirao, Nucl. Acids Res., 2005, 33, e129.
- Y. Hikida, M. Kimoto, S. Yokoyama, and I. Hirao, Nat Protoc, 2010, 5, 1312-23.
- M. Kimoto, R.S. Cox, 3rd, and I. Hirao, Expert Rev Mol Diagn, 2011, 11, 321-31.
Intellectual Property
This product is covered by patents or patents pending owned by TagCyx Biotechnologies. Purchase of this product includes a limited license to use this product solely for research. This license specifically excludes: (a) therapeutic or diagnostic applications (including products or services that incorporate this product), (b) any in vivo toxicity/safety study in support of an investigational new drug application (or foreign counterpart), (c) resale, or (d) gene functionalization activities (including products or services that incorporate data derived from gene functionalization activities) if such activities have commercial application. All of the above require a separate license from TagCyx Biotechnologies. Neither this product nor any product created through its use may be used in human clinical trials.
Ordering Information
The items in this article have been discontinued.
- Glen Report 23.11: DNA Methylation Revisited
- Glen Report 23.12: New Products Prevent Branching at Secondary Amines using DCI Activator
- Glen Report 23.13: New Products – Click Chemistry Update
- Glen Report 23.14: New Product – Ultrafast Photo Cross-Linker
- Glen Report 23.15: Product Update - Unnatural Base Pairs
- Glen Report 23.16: Technical Brief - Labelling Carboxy-Modifiers with Multiple Reporter Molecules
- Glen Report 23.17: Technical Brief - Chemical Phosphorylation – Considering the Options

