Through our collaboration with baseclick GmbH, Glen Research has been introducing a selection of products and reagents for use with click chemistry. In this update to an earlier Glen Report article,1 we cover the additional nucleoside products that we have available, add an interesting azide product, and detail a procedure suitable for click chemistry on a 1 µmole synthesis scale.
Since the launch of our collaboration with baseclick, we have had available for sale C8-Alkyne-dT-CE Phosphoramidite (1) and C8-TIPS-Alkyne-dC-CE Phosphoramidite (2) for copper(1)-catalyzed azide alkyne cycloaddition (CuAAC) click reactions.2,3 We are now happy to add C8-TMS-Alkyne-dC-CE Phosphoramidite (3), C8-TMS-Alkyne-dT-CE Phosphoramidite (4), and C8-Alkyne-dC-CE Phosphoramidite (5). C8-TIPS-Alkyne-dT-CE Phosphoramidite (6) makes up the complete family of pyrimidine derivatives for click, click-click, and click-click-click applications.4,5 This set of reagents, therefore, allows sequential and specific reactions of alkynes on column, after oligo deprotection, and after removal of the TIPS protecting group.



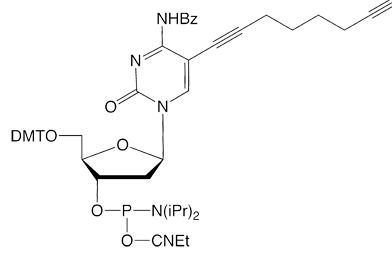
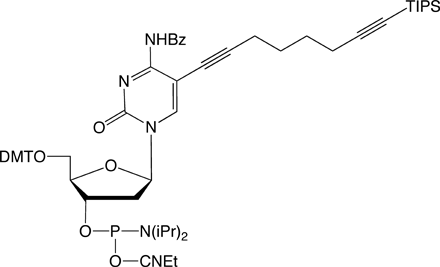
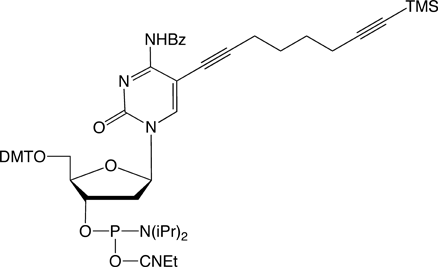
With our selection of azides, our goal is to expand the library of products available for oligonucleotide labelling rather than to duplicate products in our existing catalog. We are specifically interested in labels that are incompatible with DNA synthesis or deprotection, or labels where click chemistry offers a clear advantage over amine-NHS ester conjugation.
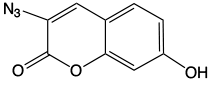
7-Hydroxycoumarin, also known as umbelliferone, is a highly fluorescent, pH-sensitive fluorophore that emits in the blue region of the spectrum. However, its fluorescence is strongly quenched if the hydroxyl is alkylated or phosphorylated, making it useful in high-throughput screening for phosphatases and lipases. Interestingly, it was found that the 3-azido derivative (7) is also highly quenched but, upon reaction with an alkyne in the presence of copper to form the triazole, the fluorescence is restored.2 The clicked coumarin emits at a lambda max of 480 nm and absorbs at 358 nm. Also, the fluorescence of the clicked coumarin is approximately 15 times higher than that of the coumarin azide (Figure 2, Page 4). This opens up the possibility to monitor a Copper-catalyzed Click reaction in real time and the detection of DNA in cells without requiring washing steps to remove the unreacted Coumarin Azide.6

The CuAAC reaction between azides and alkynes has become a very popular method for conjugation. This is because of the mild conditions employed and its orthogonal nature, which allows specific conjugations to be performed in the presence of practically any other functional groups.
One issue that we have had with this versatile reaction is the work-up after the click conjugation has been performed. The TBTA copper chelator is not water soluble nor is the CuBr. While this is not an issue with small-scale reactions, difficulties arise with large-scale reactions (~1 µmole or more). On a larger scale, simple dilution with water followed by desalting has not given us consistent results. We therefore have been working on a more robust procedure that is compatible with large-scale click reactions.
We found that the clicked oligo could conveniently be ethanol precipitated directly from the Click Reaction solution. In addition, we found that degassing the solvents prior to the Click Reaction prevented precipitation from occurring during the conjugation. This is easily accomplished by applying low vacuum to the solvents for just 2-3 minutes with gentle agitation.
References