Glen Report 22.15: New Product - A dG Amino-Modifier with Improved Hybridization Characteristics
In 2006, we introduced Amino-Modifier C6 dG (1) to complete our repertoire of amino-modified base analogs. However, we found that the placement of the linker at the C8 position led to a significant destabilization of the duplex, with the Tm dropping by 3 °C with a single incorporation. While the analog was still functional and did base pair specifically with dC, the drop in the duplex stability meant it was not a transparent substitution for dG in a sequence.
While working on an unrelated project investigating base analogs that could be used for Tm leveling (such as N-Ethyl-dC), we found the melting temperature of the C-G base pair to be remarkably insensitive to modifications at the N2 position of dG. For this reason, we decided to place the C6 alkylamine at the N2 position and test the analogue (2) in melting experiments.
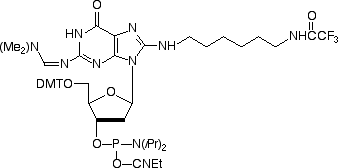
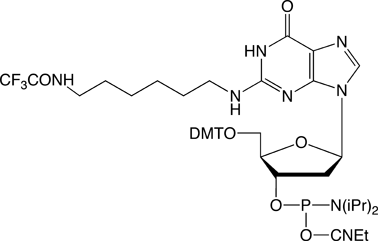
The results were quite positive. The sequence 5'-ATC XCT CAT GAT G-3' was synthesized where X = N2-Amino-Modifier C6 dG (2). After deprotection and then trityl-on purification on a Glen-Pak cartridge, the melting curve was determined when annealed to a perfectly matched reverse complement, as well as against a G/A mismatch. These results were compared to a dG control (Figure 2). The melting temperatures of the N2-Amino-Modifier C6 dG and the Control dG oligos were almost identical, with the N2-Amino-Modifier C6 dG having just a slightly higher Tm than the dG control, as shown in Figure 2. The specificity toward dC by the N2-Amino-Modifier C6 dG is maintained, with the ?Tm (Tm (match) - Tm (mismatch)) being 15 °C for the N2-Amino-Modifier C6 dG and 14 °C for the dG control.
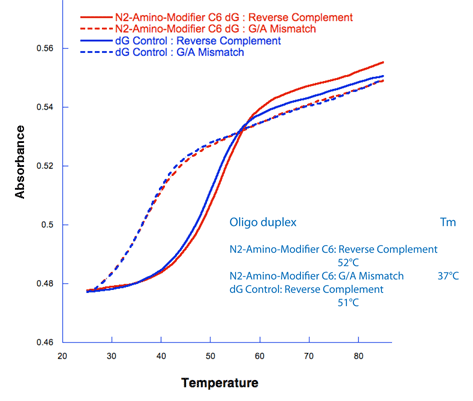
The melting curves for the amino-modified dG probe 5'-ATC XCT CAT GAT G-3', where X = N2-Amino-Modifier C6 dG, when annealed to the perfectly matching reverse complement 5'-CAT CAT GAG CGA T-3' and the G/A mismatch 5'-CAT CAT GAG AGA T-3' at 1.25 'M in 0.1 M Tris-HCl pH 7. These are compared to the dG control probe with the sequence 5'-ATC GCT CAT GAT G-3'.
As with all the trifluoroacetyl-protected amino-modifiers, we recommend deprotection in AMA to reduce side reactions that can lead to capping of the amine. To maintain conjugation efficiency for amino-modified oligos deprotected in AMA, we recommend desalting the oligo to convert it to a non-nucleophilic salt, such as Na+ or TEAH+, prior to conjugation with NHS esters or equivalents.
We are happy to provide this new N2-Amino-Modifier C6 dG to the research community.
Product Information
- Glen Report 22.11: Simple Oligonucleotide Modification using Click Chemistry
- Glen Report 22.12: The copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC)
- Glen Report 22.13: tC, tCo and tCnitro: Tricyclic Cytosine Probes of DNA and RNA Structure
- Glen Report 22.14: Saccharin 1-Methylimidazole - An Activator for DNA and RNA Synthesis
- Glen Report 22.15: New Product - A dG Amino-Modifier with Improved Hybridization Characteristics
- Glen Report 22.16:; New Products – α-Tocopherol-TEG Phosphoramidite and 3'-Thiol-Modifier 6 S-S CPG
- Glen Report 22.17: New Products - 8-Oxo-G Clamp and 5'-Amino-Modifier TEG CE Phosphoramidites
- Glen Report 22.18: Deprotection – Volume 4 – Alternatives to Ammonium Hydroxide
- Glen Report 22.19: Technical Brief - Depurination
- Glen Report 22.110: Glen-Pak™ Product Update: New Protocols for Desalting and Purification

