Authors: Antonio Manetto, Simon Warncke and Thomas Frischmuth
Address: baseclick GmbH, Bahnhofstrasse 9 – 15, 82327 Tutzing, Germany, email: [email protected]
Note: All products of baseclick are patent protected and available from Glen Research Corporation, 22825 Davis Drive, Sterling, VA 20164, USA, email: [email protected], in collaboration with baseclick.
The copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) is the most prominent example of a group of reactions named click-reactions (Figure 1, Page 2). According to Sharpless`s definition, these reactions are characterized by high yields, mild reaction conditions, and by their tolerance of a broad range of functional groups.1 Typically, the reactions require simple or no workup, or purification of the product. The most important characteristic of the CuAAC reaction is its unique bio-orthogonality, as neither azide nor terminal alkyne functional groups are generally present in natural systems.
The use of this method for DNA modification has been somewhat delayed by the fact that copper ions damage DNA, typically yielding strand breaks.2 As these problems have now been overcome by the use of copper(I)-stabilizing ligands (e.g., tris(benzyltriazolylmethyl)amine, TBTA3), Carell et al. and Seela et al. discovered that the CuAAC reaction can be used to functionalize alkyne-modified DNA nucleobases with extremely high efficiency.4


| (1) C8-Alkyne-dT | (2) C8-Alkyne-dC | (3) C8-Alkyne-7-deaza-dG | (4) C8-Alkyne-7-deaza-dA |
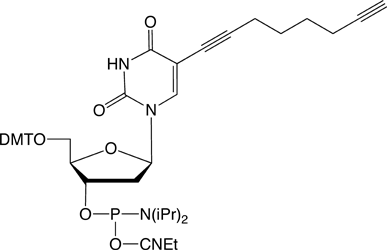
It has been shown that the 5-position of pyrimidine and the 7-position of 7-deazapurine nucleosides are the ideal positions to introduce functionalities, as these sites lie in the major groove of the DNA providing steric freedom. In order to enable efficient click-chemistry labelling of alkyne modified oligonucleotides, our nucleosides provide a 5-(octa-1,7-diynyl) side chain. Phosphoramidites of nucleosides 1-4 (Figure 2, Page 2) were shown to be incorporated into DNA oligomers by solid-phase synthesis with excellent coupling efficiency (e.g., 1: > 99 %). Another feature of the octadiynyl side chain is its stabilizing effect on DNA duplexes (e.g., 1: Tm increase of 1-2 °C).
Purified oligonucleotides bearing a single alkyne moiety are usually modified with 2-5 equivalents of the corresponding marker-azide (e.g., fluorescent-dye azides). After the addition of precomplexed Cu(I), complete conversion to the labeled oligo is observed in a time span between 30 min and 4 hours. After a simple precipitation step, labeled oligonucleotides can be recovered in near quantitative yields. On this and the following page, some examples of MALDI-mass spectra measured directly after the click reaction and the precipitation step, without further purification are presented.

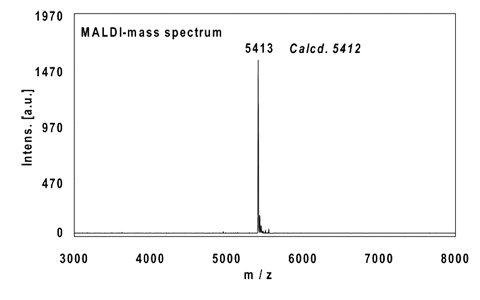
Example 1:
16mer, internal alkyne reacted with 2 equivalents Eterneon-(480/635)-Azide, 3 h at 37 °C.
Ethanol precipitation with 98% recovery of the labelled oligo.
MALDI-mass analysis of the crude product à 100% oligo-dye conjugate.
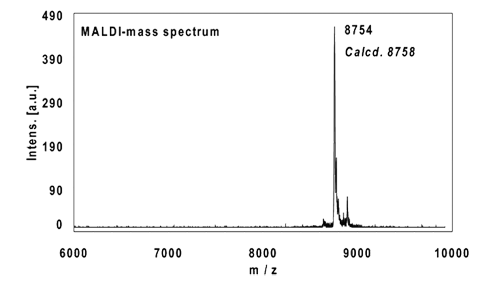
Example 2:
16mer, internal alkyne reacted with 2 equivalents Fluorescein-Azide (FAM-Azide), 3 h at 37 °C.
Ethanol precipitation with 99% recovery of the labelled oligo.
MALDI-mass analysis of the crude product à 100% oligo-dye conjugate.
The Cu(I)-catalyzed Huisgen reaction enables the multiple post synthetic labelling of alkyne modified DNA as well. Complete high-density functionalization of several alkyne moieties can be achieved without the formation of by-products.
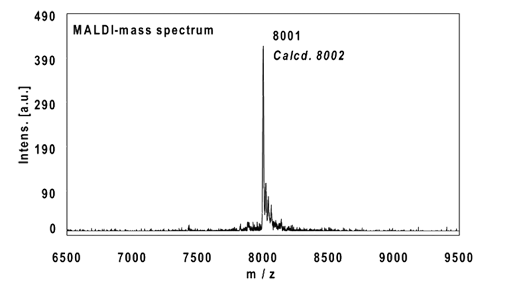
Example 3:
22mer, five internal alkynes reacted with 5 equivalents Biotin-Azide, 4 h at 37 °C.
Ethanol precipitation with 99% recovery of the labelled oligo.
MALDI-mass analysis of the crude product à 100% oligo-dye conjugate.
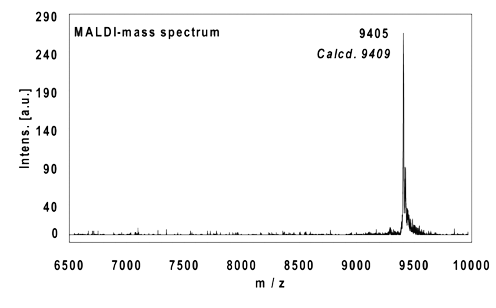
Example 4:
22mer, five internal alkynes reacted with 5 equivalents PEG-Azide, 4 h at 37 °C.
Ethanol precipitation with 86% recovery of the labelled oligo.
MALDI-mass analysis of the crude product à 100% oligo-dye conjugate.
Example 5:
22mer, five internal alkynes reacted with 5 equivalents Eterneon-(350/430)-Azide, 4 h at 37 °C. Ethanol precipitation with 85% recovery of the labelled oligo. MALDI-mass analysis of the crude product à 100% oligo-dye conjugate.
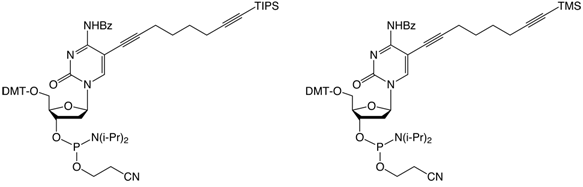
| (5) C8 -TIPS-Alkyne-dC-CEP | (6) C8-TMS-Alkyne-dC-CEP |
For the attachment of up to three different labels, phosphoramidites 5 and 6 have been developed. The alkyne groups are protected, respectively, with triisopropylsilyl (TIPS) and trimethylsilyl (TMS) protecting groups.5
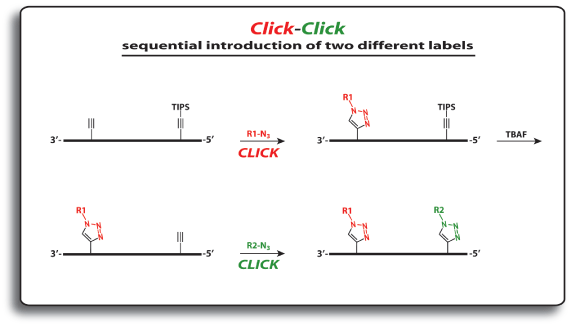
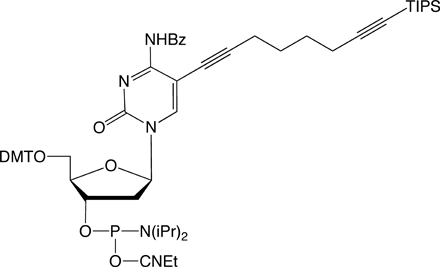
In order to modify oligonucleotides with two sensitive molecules, nucleosides 1 and 5 are incorporated into DNA strands using standard phosphoramidite chemistry. The first click reaction yields the singly modified oligonucleotide with full retention of the TIPS protecting group. For the second click, the TIPS protecting group is cleaved with tetrabutylammonium fluoride (TBAF) without causing any damage to the DNA. The second click reaction in solution yields the doubly modified oligonucleotides in excellent yields (60–90% over three steps, see picture below).
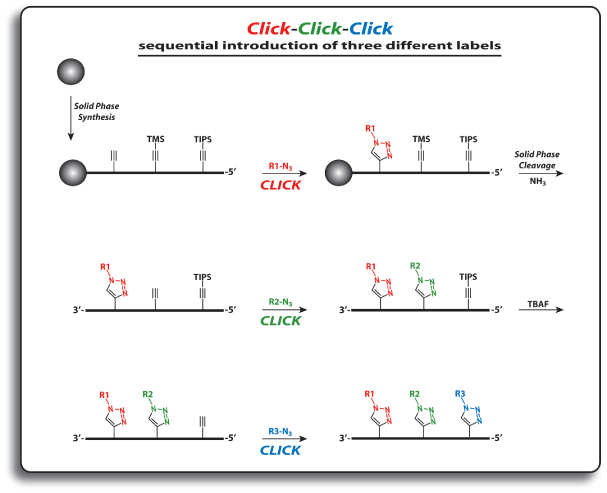
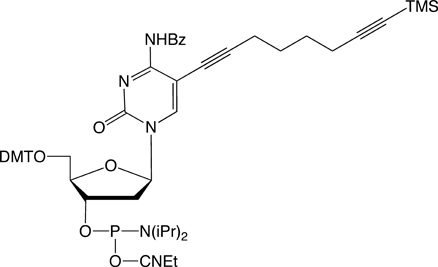
For the introduction of three different labels, nucleosides 1, 5 and 6 are introduced into oligonucleotides. The first click reaction is performed directly on the resin. The singly modified oligonucleotide is subsequently cleaved from the support under concomitant cleavage of the TMS group and retention of the TIPS protecting group. The second click reaction is performed in solution. Precipitation of the doubly modified oligonucleotide, cleavage of the TIPS group with TBAF, and a subsequent third click reaction in solution furnishes the desired triply modified oligonucleotides in excellent overall yields (see picture on next page).
In comparison to the common post synthetic labelling methods of oligonucleotides like amine/NHS-ester, thiol/iodoacetamide or maleimide labelling, modification of oligonucleotides with click chemistry is providing by far the highest conjugation efficiency.6 Single and multiple labelling can be performed with as little as two equivalents of label-azides resulting in complete conversion and high yields of labeled oligo. Alkyne-modified nucleoside phosphoramidites are incorporated into DNA strands during solid-phase synthesis in excellent yields, even stabilizing the DNA-duplexes. In addition, the label-azides used for click functionalization are stable to hydrolysis (in contrast to sensitive NHS esters and maleimides) and excess amounts can be recovered after the click reaction.
The easy-to-use copper(I)-catalyzed azide-alkyne cycloaddition reaction with its outstanding selectivity is in an excellent position to take over as the state-of-the-art methodology to label and modify DNA and nucleic acids in general.
Baseclick GmbH has filed the following patent applications: