The sensitivity in quantum yield to immediate surroundings is a common feature for the fluorescent base analogues that were developed - until very recently. This sensitivity normally gives rise to considerable changes in quantum yield (up to 100 times). These changes can successfully be used for monitoring, for example, DNA hybridization/melting, nucleic acid conformational changes and nucleic acid-protein interactions. Glen Research offers important fluorescent base analogues, such as 2-aminopurine1 and pyrrolo-C2, that are sensitive to their microenvironment. Now, with technical and scientific support from ModyBase HB, we have added to our catalog two new fluorescent base analogues, tC and tCO (Figure 1), which are virtually insensitive to their microenvironment. These analogues were developed by the researchers that founded ModyBase HB.
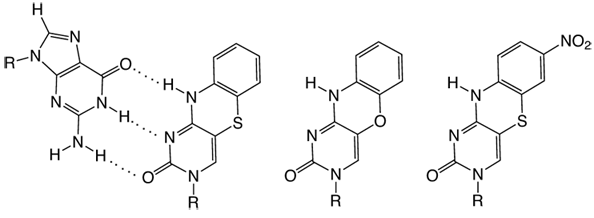
In contrast to previous fluorescent base analogues, the tC products have been shown to be virtually insensitive to their immediate surroundings in single- and double stranded (tC) and double stranded systems (tCO), respectively.3-5 It has been shown in extensive investigations that these fluorophores work excellently as analogues of cytosine and only give minor perturbations to the overall structure of the nucleic acids they are incorporated into.4-7 For example, they leave duplex DNA in the natural B-form and increase the Tm of 10-mers by on average 3 °C.4-6
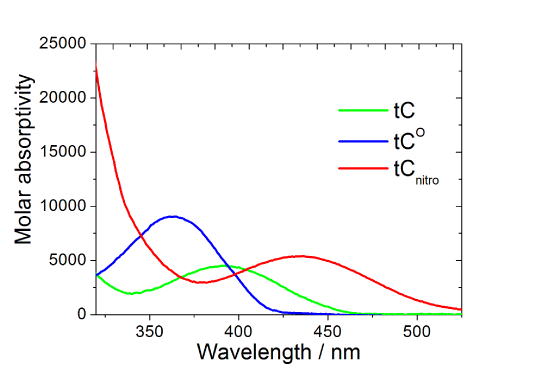
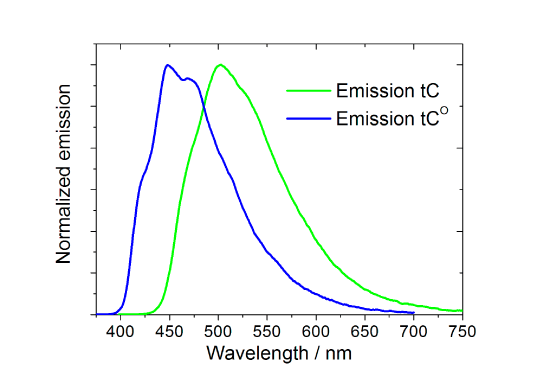
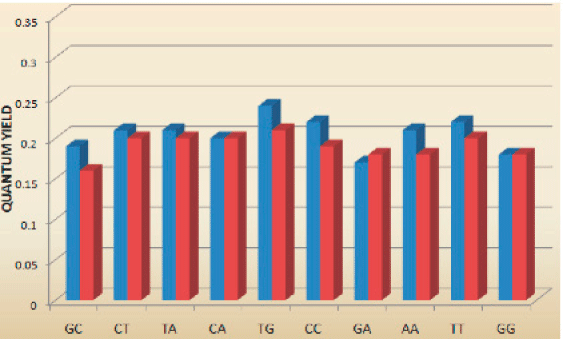
The spectral properties of tC (λexc=395 nm, λem=505 nm) and tCO (λexc=365 nm, λem4-5. Interestingly and surprisingly, both tC and tCO have, as mentioned above, virtually insensitive quantum yields in double stranded DNA ranging from 0.16 to 0.21 and 0.17 to 0.27 (Figure 3, Page 6), respectively.4-5 Furthermore, there is basically no change when going from single- (?f=0.17-0.24; Figure 3, Page 6), to double-stranded systems containing tC.4 Moreover, the fluorophores display single lifetimes (tCduplex≈6.3 ns, tCss≈5.7 ns, tCO?duplex≈4.1 ns) under the conditions mentioned.4-5

These properties of tC and tCO in combination with the high control of their orientation and position within the nucleic acid base stack make them unique among fluorescent base analogues and particularly well-suited for fluorescence resonance energy transfer (FRET) and fluorescence anisotropy experiments. As will be described in detail below, these properties are vital in accurately determining the Förster distance (R0) and, thus, also for the precision of distance (RDA) estimates using FRET. Moreover, a single lifetime and bright emission, in combination with a firm stacking and a low rate of base-flipping, are crucial properties for detailed studies of the local dynamics of nucleic acids using fluorescence anisotropy.5-6
In what may be the largest step forward in the development of fluorescent base analogues since 2-aminopurine was presented in the late 60s,1 the base analogue tCnitro (Figure 1) was presented as a FRET-acceptor in 2009 together with tCO (or tC) as the donor molecule.8 This constitutes the first ever description of a nucleobase FRET-pair. Thorough examination of the photophysical properties (Figure 2), including spectral resolution into distinct transition dipole moments, has also been performed to allow future detailed nucleic acid structure determinations using FRET.9
As discussed above, tCO (and tC) have several properties that make them ideally suited as FRET-donors in nucleic acid systems:
The first three properties are important in order to have a high control of the Förster distance (R0=0.211(JDAκ2n-4φD)1/6 in Å), which in turn enables very accurate distance determinations (RDA, distance between the donor and acceptor) using FRET. This novel FRET-pair provides a unique tool for investigations of nucleic acid containing systems. It also makes it possible to study nucleic acid structure and to monitor structural changes with a very high level of detail both in distance and orientation over more than one turn of the DNA helix (see Figure 4).8-9

In addition to the interesting properties of tC and tCO already mentioned, these fluorophores are on average the brightest fluorescent base analogues when incorporated into duplex DNA.5 With a molar absorptivity of 9000 M-1 cm-1, the average brightness of tCO is almost double that of tC. Furthermore, compared to other fluorescent base analogues that are highly quenched inside DNA, tCO has a brightness that is up to 50 times higher on average in dsDNA. With a quantum yield change reaching approximately a factor of 2 between single and double strands and no influence on Tm for wisely chosen neighboring bases, this high brightness makes tCO interesting not only in duplex FRET and fluorescence anisotropy measurements but also highly attractive as a probe of nucleic acid secondary structure changes.10
The tC-family has already been utilized in several biophysical, biochemical and biological investigations. In a recent anisotropy study, it was shown that tC and tCO work excellently as probes for directly monitoring motion of nucleic acid helical structures, rather than a combination of motion of the overall structure and the probe itself, as is the case for most of the currently available fluorescent base analogues.5 Furthermore, tC has been utilized as a FRET-donor in pair with rhodamine in a PNA-DNA-hybrid,3 and in pair with Alexa-555 in a study of conformational dynamics of DNA polymerase.11
In other polymerase studies, it was shown that the 5'-triphosphates of tC and tCO are efficiently incorporated into DNA by the Klenow fragment although with increased frequency of mutations.12-13
In addition to these DNA polymerization investigations, the ribonucleotide form of tC has been synthesized and tested as a substrate for T7 RNA polymerase.14 Interestingly, it was found in this study that T7 RNA polymerase does not misincorporate tC and it was shown that the ribonucleotide form of tC could replace normal cytosine in a ∼800 nucleotide RNA, enabling straightforward fluorescent labelling of long RNAs.
Finally, it has been established that the change in quantum yield of tCO between single and double stranded systems can be utilized for the detection of individual melting processes of complex nucleic acid structures.10
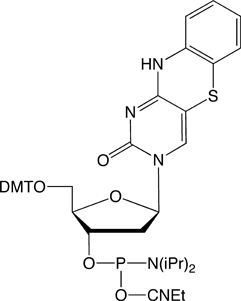
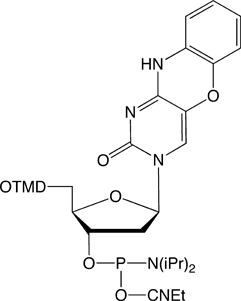
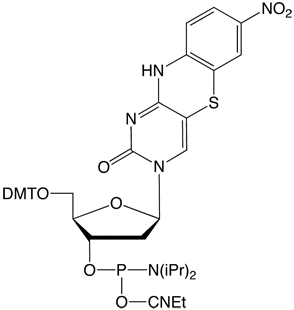
Previously, the use of the tC-family of molecules was possible only because tC/tCO/tCnitro were synthesized in the labs of individual researchers. Glen Research is proud to offer these products for sale and now make it possible for the whole research society to use these interesting probes.
We thank Marcus Wilhelmsson, Department of Chemical and Biological Engineering/Physical Chemistry, Chalmers University of Technology, Göteborg, Sweden for his immense help in preparing this article.
These products are offered under license from Isis Pharmaceuticals, Inc. and in collaboration with ModyBase HB.
tC-CE Phosphoramidite (10-1516)
tCo-CE Phosphoramidite (10-1517)
tCnitro-CE Phosphoramidite (10-1518)