Glen Report 19.19: NHS-Carboxy-dT – Expanding the NHS Ester Phosphoramidite Repertoire
Introduction
When Glen Research was founded in 1987, the oligonucleotide market was very different. In these days, researchers were just delighted to have ready access to unmodified DNA oligos at a reasonable price but, in our view, the biggest change has been the adoption of strategies that use modified oligonucleotides and the ready availability of these oligos. Modification with amines and thiols at the 3' and 5' termini, as well as within the sequence, have supported explosive growth in fields such as labeling and array construction.
The most common strategy is for oligonucleotides to be modified with a nucleophile – e.g., amine or thiol – and then reacted with the corresponding N-hydroxy-succinimide (NHS) ester or maleimide of the desired label. However, the opposite approach can also be taken in which the oligonucleotide is modified with an electrophilic NHS ester and is conjugated while still on the support with a nucleophile – such as an amino-, hydrazide-, or hydroxylamine-modified label (On-Column labeling). Afterward, the labeled oligonucleotide is cleaved from the support and deprotected. In 2002, we introduced the Carboxy-Modifier C10 (1) which is the phosphoramidite derivative of an N-hydroxysuccinimidyl (NHS) ester of a decanoic acid. This has proven to be a popular item for the greater flexibility it gives the researcher.
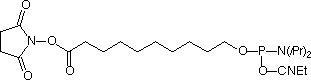
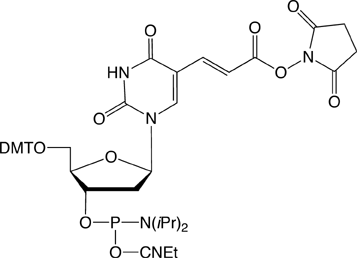
On-Column Labeling
There are a number of advantages to On-Column labeling of oligonucleotides. Foremost is probably the much-enhanced speed and convenience of the conjugation which is amenable to high-throughput production of labeled probes. For example, as a model system, we used Dansyl Cadaverine in DMSO containing 0.5% diisopropylethylamine to label oligonucleotides quantitatively in less than 2.5 minutes at room temperature. The unreacted Dansyl Cadaverine was conveniently rinsed from the column – a great improvement over time-consuming and labor-intensive desalting of an oligo over a Glen Gel-Pak™ or Sephadex column.
A further advantage of On-Column labeling is the minimal amount of label that is required to conjugate an NHS-derivatized oligonucleotide. This is especially useful when conjugating with an expensive molecule or antigen. With as little as 1.3 equivalents, we have observed quantitative labeling in less than 2 hours at room temperature. The reason is straightforward – in standard solution phase NHS ester chemistry, the reaction of the NHS ester with the primary amine is competing against the hydrolysis of the NHS ester since the reaction is typically performed in aqueous buffer at pH 9. As a result, On-Column labeling uses roughly 20-25% of the amount of label required for solution-phase conjugation reactions. Furthermore, with On-Column labeling, the unreacted amino-modified label can be collected and reused for further conjugations.
The only caveat for On-Column labeling is that the conjugated label HAS to be able to withstand the deprotection and DNA synthesis chemistries without degradation or branching off the label.
Given these useful qualities, we have decided to expand our repertoire of NHS ester derivatives to include the NHS-Carboxy-dT Phosphoramidite (2). By making a dT analog of the Carboxy-Modifier C10, it is possible to label one or multiple sites within an oligonucleotide. This opens up the possibility to label any number of different dyes or molecules within an oligonucleotide when the phosphoramidite is unavailable. Doing so is straightforward and may be done manually off the synthesizer or even in a fully-automated manner on the DNA synthesizer.
An obvious question is whether the synthesis of the oligo could be completed and then the NHS-Carboxy-dT residues within the sequenced labeled. The answer is yes – but the results are mixed. After the NHS-Carboxy-dT has undergone an additional 18 coupling cycles, approximately 50% of the NHS ester has been hydrolyzed. We believe that this is primarily due to hydrolysis during oxidation. For this reason, we recommend using a low-water content oxidizer during synthesis, such as 40-4032-xx, which we have found improves the stability of the NHS ester.
Manual Labeling
For a manual coupling of the NHS-carboxy-dT off the synthesizer, the synthesis is paused after the addition of the NHS-carboxy-dT. The column is removed and fitted with two syringes, one containing the amino-modified label dissolved in an aprotic solvent such as ACN, DMF or DMSO containing 0.5 – 1.0% diisopropylethylamine. The solution is pushed back and forth and then allowed to sit for typically three to fifteen minutes, depending upon the label and its concentration. After the conjugation is complete, the column is rinsed with fresh solvent and given a final rinse with anhydrous ACN. The column is then placed back on the synthesizer and the synthesis resumed.
Automated Labeling
For the automated conjugation of the NHS-Carboxy-dT, it is necessary to edit the synthesis cycle such that the NHS-Carboxy-dT is labeled just after its incorporation into the oligonucleotide. The most straightforward way to do this is to put the amino-modified label in a reserve port that is only activated during the coupling of the NHS-dT within the sequence.
Automated Protocol
For an ABI 394, for example, the NHS-Carboxy-dT phosphoramidite can be placed on port 6 and the amino-modified label in port 7. In the modified cycle, Function 31 ‘7 to column’, can be inserted and selected to be active only when the NHS-carboxy-dT on port 6 is being coupled. Similarly, on an Expedite 8909, the NHS-Carboxy-dT phosphoramidite can be placed on ports 5-8 and the label on the auxiliary port. Cycle 5-8 (wherever the NHS-Carboxy-dT is placed) can be modified so that the Function 17 ‘Aux to column’, can be inserted after the standard oxidation. After an appropriate waitstep of approximately 2.5-15 minutes, the unreacted label is rinsed from the column and the synthesis proceeds to the next base.
A copy of the modified ABI 394 cycle used can be found at NHS-labeling-cycle.pdf
On-Column Dansyl Labeling
To confirm this On-Synthesizer labeling technique, the following oligonucleotide was synthesized on an ABI 394.
5’-6TT T6T TT6 TT-3’
where 6 = NHS-Carboxy-dT. After each incorporation of the NHS-Carboxy-dT, Dansyl Cadaverine was pulled from port 7 and allowed to couple for 5 minutes. After the synthesis was completed, the oligo was deprotected in 30% Nh4OH and analyzed by RP HPLC, as shown in Figure 1. Analysis by Electrospray MS confirmed quantitative labeling by the Dansyl Cadaverine.
On-Column Cystamine Labeling
The NHS-Carboxy-dT phosphoramidite also allows for other interesting and useful modifications for which there are no base analogs available. For instance, labeling with Cystamine hydrochloride can yield an internal thiol modifier to be used for cross-linking. Shown in Figure 2 is an example of a manual conjugation of Cystamine with an end-labeled NHS-dT. Upon reduction with DTT, a single peak was observed, indicating quantitative labeling.
These reaction schemes are detailed in Scheme 1 on Page 15. In addition, the reaction with propargylamine is shown to demonstrate the potential application to 'click' chemistry, as detailed on Page 9.
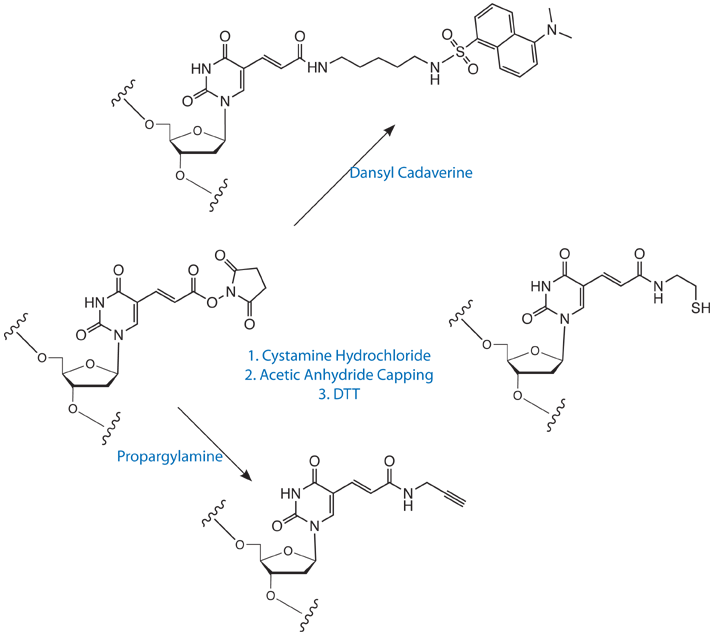


1) The chromatogram of NHS-Carboxy-dT-T5 labeled with Cystamine-2HCl after reduction with DTT. Cystamine-2HCl (15 mg) was dissolved in 1 mL DMSO with 35 µL DIEA added and allowed to react for 15 minutes at room temperature. After cleavage and deprotection in ammonium hydroxide, the disulfide was reduced with DTT and the oligo analyzed by RP HPLC. The earlier eluting peaks are DTT and oxidized DTT respectively. 2) The control oligo with the NHS-Carboxy-dT hydrolyzed with 1 M NaOH 15 minutes at 65 C. Note, when labeling an oligo internally with cystamine, the oligo must be capped with acetic anhydride/N-methylimidazole after the cystamine conjugation to prevent branching off the second amine.
Conclusion
This technique opens up the possibility of automating the synthesis of DNA probes labeled even with dyes that usually require post-synthesis labeling. A good example would be the popular Alexa™ dyes which are not compatible with regular oligonucleotide synthesis. However, these dyes are all available as either the cadaverine or hydrazine derivative (http://probes.invitrogen.com/handbook/tables/0728.html) and one could easily envisage automated high-throughput production of probes based on these dyes. In another example, labeling with propargylamine would offer an internal alkyne for Cu(I)-catalyzed 'click' chemistry conjugation with an azide. The possibility even exists to develop procedures for conjugating oligos with peptides and PNA derivatives.
Product Information
- Glen Report 19.11: Modified RNA Phosphoramidites Useful in siRNA Research and Biologically Significant 1-Methyl-adenosine
- Glen Report 19.12: 1-Methyl-Adenine in Nucleic Acids
- Glen Report 19.13: Technical Brief - Precautions During Packaging of Phosphoramidites and Transfer into Alternative Vials
- Glen Report 19.14: Microarrays, Nanotechnology and Beyond
- Glen Report 19.15: 5’-Hexynyl Phosphoramidite – Conjugation with a Click
- Glen Report 19.16: Quenched Autoligation (QUAL) Probes
- Glen Report 19.17: Selenium Derivatization of Nucleic Acids for X-ray Crystal Structure Determination
- Glen Report 19.18: Large Scale Synthesis Update
- Glen Report 19.19: NHS-Carboxy-dT – Expanding the NHS Ester Phosphoramidite Repertoire

