Glen Report 19.15: 5’-Hexynyl Phosphoramidite – Conjugation with a Click
The ability to perform a specific conjugation in biological samples is a daunting task. The presence of the complex variety of functional groups leads to almost unavoidable cross-reactivity and non-specific labeling. However, one reaction which shows remarkable selectivity is the [3+2] cycloaddition between azides and alkynes, as shown in Figure 1.
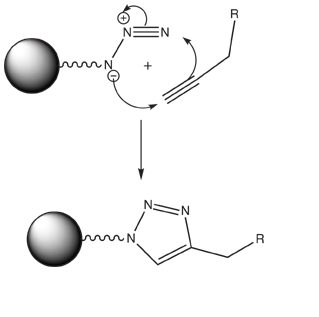
Huisgen originally described the reaction of azides and alkynes to form 1,2,3-triazoles,1 but the incredible utility of the reaction was not borne out until Sharpless reported2 that the addition of copper (I) iodide (CuI) led to a million-fold increase in the rate of the cycloaddition. The reaction was found to be so exquisitely regioselective and efficient at even the most mild conditions that Sharpless coined the term ‘Click Chemistry’ to describe it. This chemistry has been used to label specific proteins from cell lysates with essentially no nonspecific labeling3 and can even be coaxed to occur at specific locations in a microarray with 10 micron resolution by applying a potential of -300 mV.4
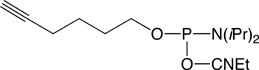
Given the potential of this chemistry, we are happy to introduce the 5’-Hexynyl Phosphoramidite (1). Oligonucleotides containing the 5’-Hexynyl moiety are stable to standard deprotection conditions and exhibit a slightly increased retention time on RP HPLC. A number of azide labels are commercially available for click conjugation to a 5'-hexynyl-modified oligonucleotide.
Azide-modified labels and tags are commercially available, but how easy is it to access azido-labeled oligonucleotides? Azides are not compatible with oligonucleotide synthesis using phosphoramidites so a post-synthesis displacement reaction is likely to be required. We found we were able to quantitatively convert a 5’-iodo-modified oligonucleotide (prepared using 5'-Iodo-dT) to the corresponding 5’-azide. Specifically, after synthesis of the oligonucleotide, the CPG was transferred to a vial and heated at 55 °C for 1 hour in a solution of NaN3 in DMSO (~5 mg/mL). After rinsing the CPG with DMSO and ACN, the oligo was deprotected in AMA for 15 minutes at 65 °C to afford the 5’-azido-modified oligonucleotide. When this oligo was reacted in the presence of 0.6 mM CuI in DMSO/water with the hexynyl-labeled oligo, IEX HPLC confirmed the conjugation of the two oligonucleotides.
References
- R. Huisgen, Angew Chem Int Ed, 1963, 2, 565-598.
- V.V. Rostovtsev, Green, L.G., Fokin, V.V. and Sharpless, K.B., Angew Chem Int Ed, 2002, 41, 2596-2599.
- A.E. Speers, G.C. Adam, and B.F. Cravatt, J Amer Chem Soc, 2003, 125, 4686-7.
- N.K. Devaraj, P.H. Dinolfo, C.E. Chidsey, and J.P. Collman, J Amer Chem Soc, 2006, 128, 1794-5.
Product Information
- Glen Report 19.11: Modified RNA Phosphoramidites Useful in siRNA Research and Biologically Significant 1-Methyl-adenosine
- Glen Report 19.12: 1-Methyl-Adenine in Nucleic Acids
- Glen Report 19.13: Technical Brief - Precautions During Packaging of Phosphoramidites and Transfer into Alternative Vials
- Glen Report 19.14: Microarrays, Nanotechnology and Beyond
- Glen Report 19.15: 5’-Hexynyl Phosphoramidite – Conjugation with a Click
- Glen Report 19.16: Quenched Autoligation (QUAL) Probes
- Glen Report 19.17: Selenium Derivatization of Nucleic Acids for X-ray Crystal Structure Determination
- Glen Report 19.18: Large Scale Synthesis Update
- Glen Report 19.19: NHS-Carboxy-dT – Expanding the NHS Ester Phosphoramidite Repertoire

