Glen Report 18.16: Fluorous Affinity Purification of Oligonucleotides
- A higher affinity alternative to RP cartridge purification.
- One-pass loading without ammonia removal.
- High recoveries (typically 70-100%).
- High selectivity for removal of failure sequences, even with long oligonucleotides.
- Excellent for longer oligonucleotides (e.g. 50-100+ mers); recoveries nearly quantitative.
- Does not require new techniques.
The fluorous affinity purification of oligonucleotides is a quick and simple affinity-based method for the purification of oligonucleotides that relies on the strong interaction of fluorous-tagged oligonucleotides (made with fluorous-tagged phosphoramidites, Figure 1) with the fluorinated adsorbent present in Fluoro-Pak™ columns.1 Fluorous affinity purification is operationally similar to DMT-on purification using a reverse-phase (RP) adsorbent, e.g., RP cartridge purification, except that it involves a much stronger affinity interaction and is thus able to afford higher selectivities and recoveries, even with long oligos. DMT-on RP cartridge purification is limited to relatively short oligonucleotides, typically ≤30-40-mers, since the relative hydrophobic contribution of the lipophilic DMT group diminishes as the chain length increases, resulting in lower overall yields and a diminished selectivity of the adsorbent for the desired oligonucleotide over failure sequences.
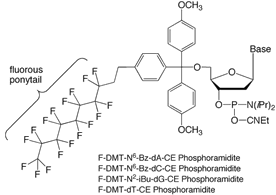
Highly fluorinated organic compounds are both hydrophobic and lipophobic, preferring instead to associate with other fluorinated substances.2 For example, perfluorohexane is insoluble in both water and hexane. Organic molecules that have both an organic domain (e.g. an oligonucleotide) and a perfluoroalkyl domain (e.g., a linear perfluoroalkyl “ponytail”) are known as fluorous molecules, and may be separated from non-fluorous molecules by interaction with fluorinated separation media such as Fluoro-Pak columns. Fluorous-fluorous interactions are strong and selective. Fluorous affinity interactions are well-documented in the chemistry literature, and are now finding application in more biological areas, e.g., proteomics3 and now oligonucleotides.1,4
The fluorous affinity purification of oligonucleotides is similar to DMT-on RP cartridge purification, and we have developed protocols that are as “plug-and-play” as possible.5 The first step is to install a single nucleotide at the 5’-terminus of the oligonucleotide using a fluorous-tagged phosphoramidite (Figure 1). The fluorous tag takes the form of a fluorous DMT group (“FDMT” group), where a fluorous ponytail is attached via an ethylene spacer to a normal DMT group. The FDMT group is designed so that it behaves just like a DMT group: the rate of detritylation is very similar to that observed for the DMT group and the absorbance maximum of FDMT cation (504 nm) is identical to DMT cation. Please note that only one coupling of a 5’-O-FDMT phosphoramidite is required; normal DMT amidites are used for the earlier steps in the synthesis. The synthesis is run in DMT-on mode, leaving the FDMT group in place. FDMT-bearing amidites are entirely soluble in acetonitrile and couple normally.
The FDMT-on oligonucleotide is cleaved from the solid support as usual and the base protecting groups are removed according to standard methods. If ammonia is used, it is not necessary to evaporate it, since Fluoro-Pak columns use a pH-stable polymeric matrix. HPLC analysis of a crude 75-mer mixture shows that the fluorous-tagged oligonucleotide is strongly retained, even on an RP-HPLC column (Figure 2).
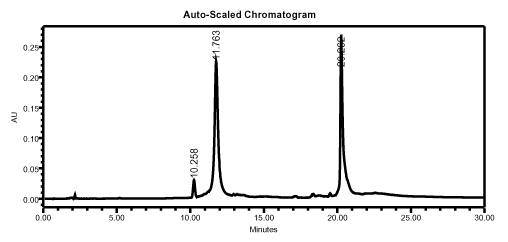
HPLC analysis of a crude 75-mer, showing that the fluorous-tagged oligonucleotide is strongly retained. Waters Spherisorb ODS-2 (5 µm, 4.6 x 150 mm, 1 mL/min), mobile phase A = 0.1 M aqueous TEAA; mobile phase B = acetonitrile.
The crude deprotection solution is diluted with a salt-containing loading buffer and applied to a Fluoro-Pak column. Binding of the FDMT-tagged oligonucleotide occurs in one pass, leaving most of the failure sequences unbound. Washing with 10% acetonitrile in 0.1 M TEAA removes the rest of the failures. Additional failure washes are unnecessary, but they reveal that the fluorous-tagged oligonucleotide is still retained; no leaching from the column is observed, even with 100-mers. Such selectivity is unprecedented with DMT-on cartridge purification. On-column detritylation with TFA followed by elution of the purified oligonucleotide is then carried out. As an example, fluorous-tagged mixed-base 75-mers (200 nmol scale) were purified by fluorous affinity purification to provide 9-11 A260 units of the fully deprotected material, free from failure sequences. This represented a nearly quantitative recovery of the available FDMT-tagged oligonucleotides present in the crude ammonia deblock solutions (estimated by HPLC). Ion-exchange HPLC of the crude 75-mer with DMT removed is shown in Figure 3. The ion-exchange chromatogram of the fluorous-purified 75-mer is shown in Figure 4. The method was extended to 100-mers, which resulted in recoveries of 76-100% of the available tagged oligonucleotides, again free of failure sequences.
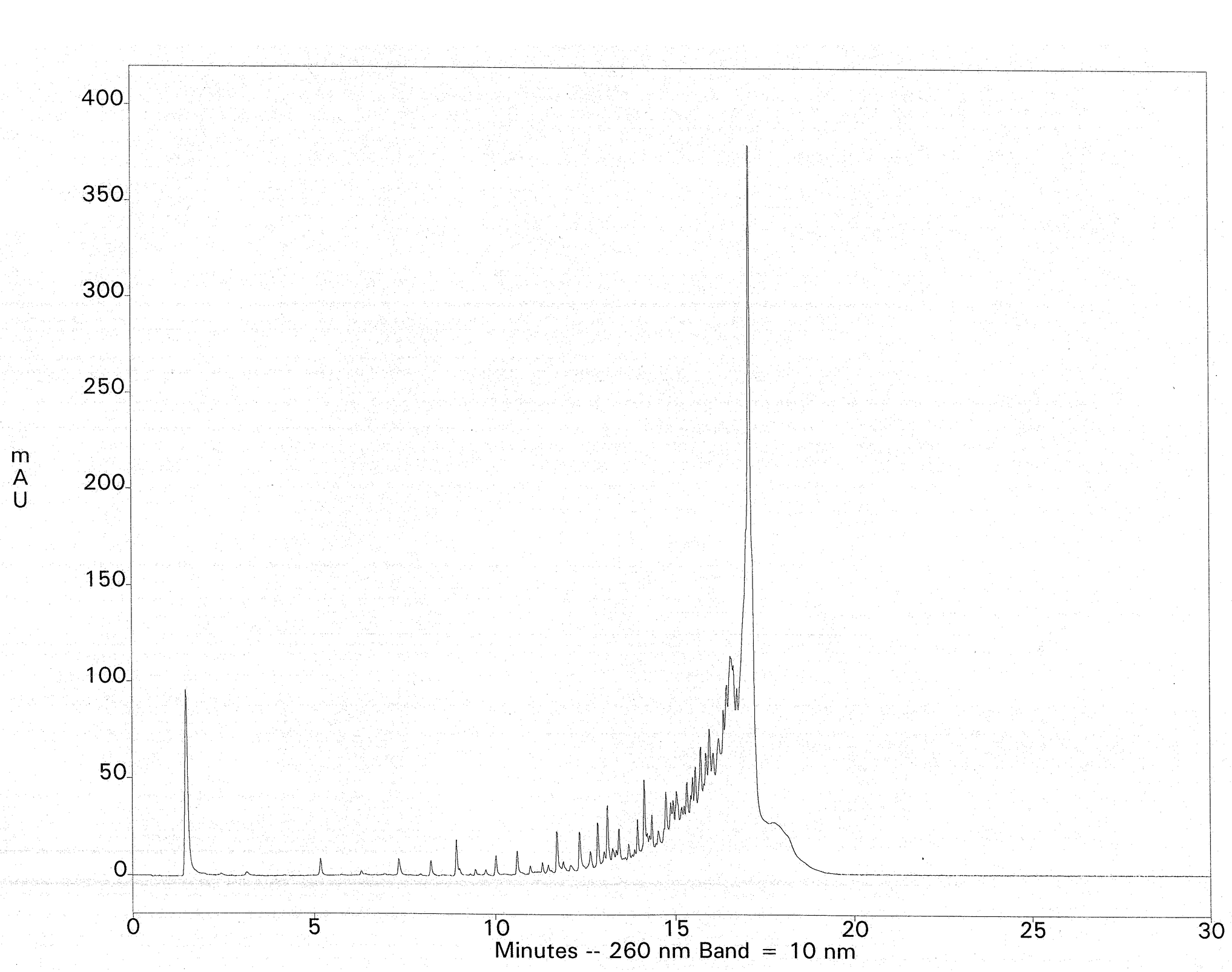
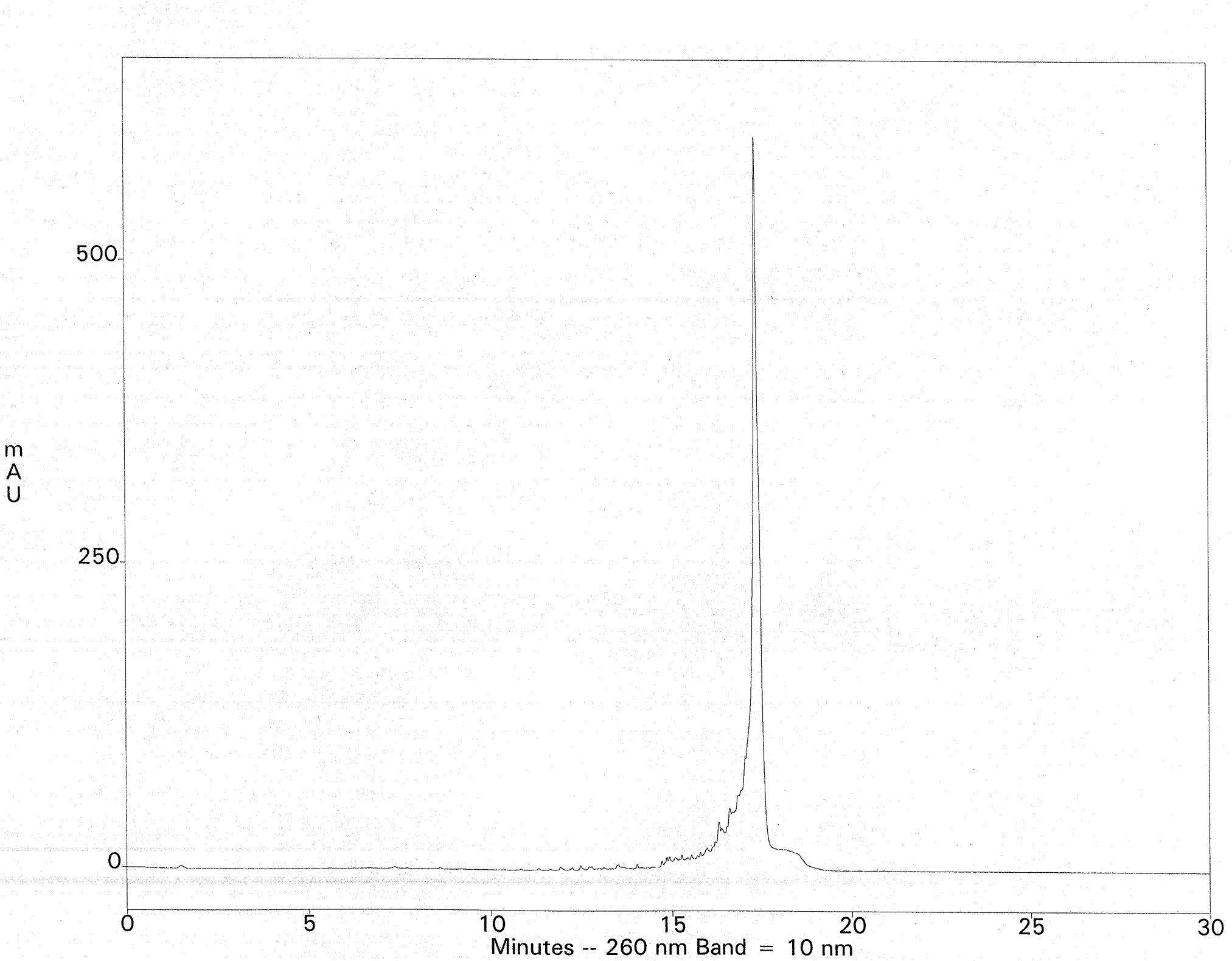
Column: Dionex DNAPac PA200, 4 x 250mm Buffers: A- 10mM NaClO4, 25mM TRIS-HCl, 20% Acetonitrile, pH 7.4 ; B- 600mM NaClO4, 25mM TRIS-HCl, 20% Acetonitrile, pH 7.4 Gradient: 0-40% Buffer B at a flow rate of 1mL/min.
Fluoro-Pak™ and Fluoro-Pak™ II Columns
Fluoro-Pak adsorbent has fluorinated organic groups bound to a pH-stable polymeric resin and is ideal for the purification of fluorous-tagged oligonucleotides. Multiple pore and particle sizes have been evaluated in order to provide optimal performance with reasonable back-pressure. Flow may be induced by pressure or vacuum. Fluoro-Pak columns should be used with fluorous-tagged oligonucleotides; they are not designed for normal DMT-on purifications. Further, Fluoro-Pak columns were designed specifically for the fluorous purification of oligonucleotides and have not been validated for other uses, although they may provide a good alternative to traditional silica-based fluorous adsorbents. Two columns are available: Fluoro-Pak Columns, containing 75 mg of adsorbent, useful for up to 0.2 micromole purifications, and Fluoro-Pak II Columns, containing 150 mg of adsorbent, useful for 1 micromole purifications.
Additional Fluorous Monomers
In addition to the basic four fluorous deoxyribonucleoside phosphoramidites, fluorous versions of other nucleic acid synthesis reagents are in development.
A significant percentage of longer oligonucleotides are phosphorylated at the 5' terminus to allow further reactions, e.g., ligation, to take place. Fluorous Chemical Phosphorylation Reagent II (F-CPR-II), the fluorous version of CPR-II, allows the combination of fluorous affinity purification with 5’-phosphorylation.6 In situations where all long oligos are required to be 5'-phosphates, F-CPR-II acts as the common fluorous tag for all oligos.
Detailed information on the fluorous purification method may be found in the booklet “User Guide: Fluorous Affinity Purification of Oligonucleotides”, which is included with each order.5
We thank Dr. Will Pearson of Berry & Associates, Inc. for helpful discussions during the preparation of this article.
Intellectual Property
“Fluoro-Pak” is a trademark of Berry & Associates, Inc. Products for Fluorous Affinity Purification of Oligonucleotides: Patents applied for, Berry & Associates, Inc. Further, the use of these products is licensed under U.S. Patents 6,673,539, 6,156,896; 5,859,247; and 5,777,121 and one or more pending patents owned or controlled by Fluorous Technologies, Inc. CPR-II is subject to US Patent 5,959,090, assigned to Glen Research Corporation.
References:
- Pearson, W. H.; Berry, D. A.; Stoy, P.; Jung, K.-Y.; Sercel, A. D. J. Org. Chem. 2005, 70, 7114-7122.
- Handbook of Fluorous Chemistry; Gladysz, J. A.; Curran, D. P.; Horváth, I. T., Eds.; Wiley-VCH: Weinheim, 2004.
- Brittain, S. M.; Ficarro, S. B.; Brock, A.; Peters, E. C. Nature Biotechnol. 2005, 23, 463-468.
- For related work, see: (a) Beller, C.; Bannwarth, W. Helv. Chim. Acta 2005, 88, 171-179 and (b) Tripathi, S.; Misra, K.; Sanghvi, Y. S. Org. Prep. Proc. Int. 2005, 37, 257-263.
- User Guide: Fluorous Affinity Purification of Oligonucleotides, 2005, Berry & Associates, Inc., http://www.berryassoc.com/literature/fluorousguide.pdf
- Guzaev, A.; Salo, H.; Azhayev, A.; Lönnberg, H. Tetrahedron 1995, 51, 9375-9384.
Product Information
The Fluoro-Pak columns have been discountinue. Please contact Glen Support;
- Glen Report 18.11: AP-dC - a Cytosine Analogue Capable of Clamp-like Binding to Guanine - G-clamp
- Glen Report 18.12: Trimer Phosphoramidites – Tools for fine-tuning protein function
- Glen Report 18.13: Sulfurizing Reagent II - Stable in Solution and Optimized for RNA Sulfurization
- Glen Report 18.14: Caps for Increased Duplex Stability and Base-Pairing Fidelity at Termini
- Glen Report 18.15: Synthesis of Branched DNA with a Comb Structure
- Glen Report 18.16: Fluorous Affinity Purification of Oligonucleotides
- Glen Report 18.17: Potent Inhibition of Cytosine-5-methyltransferases
- Glen Report 18-1 Supplement: Sulfurizing Reagent II and its use in Synthesizing Oligonucleotide Phosphorothioates

