Glen Report 18.14: Caps for Increased Duplex Stability and Base-Pairing Fidelity at Termini
Glen Research is pleased to offer two new products for the synthesis of oligonucleotides. The new products allow for the preparation of hybridization probes with increased affinity for complementary sequences. Both are phosphoramidites that can be readily introduced via automated DNA synthesis at the end of solid phase syntheses. The caps favor the formation of stable Watson-Crick duplexes by stacking on the terminal base pair (Figure 1). Melting point increases of over 10 °C per modification can be realized for short duplexes.1,2

The caps fit canonical Watson-Crick base pairs and do not stack well on mismatched base pairs. This leads to increased base pairing selectivity at the terminal and the penultimate position of oligonucleotides featuring the caps. Base pairing fidelity is usually low at the termini, where fraying occurs frequently in the absence of caps. The beneficial effects of the caps are also realized when longer target strands are bound, so there is no need for blunt ends for the duplexes formed.1,2 The caps, when attached to the terminus of an oligonucleotide, also facilitate purification as their lipophilicity leads to prolonged retention on reversed phase columns or cartridges. Finally, capping of termini may discourage the degradation of oligonucleotides by exonucleases. Figure 2 shows the structures of the phosphoramidites producing the caps.
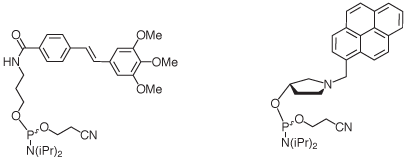 |
|
| Trimethoxystilbene (1) | Pyrenylmethylpyrrolindol (2) |
Trimethoxystilbene
Stilbenes have been successfully employed for covalently bridging the termini of oligonucleotide hairpins.3 The trimethoxystilbene cap that is now available is the result of a recent study that focused on stilbenes that are covalently linked to only one of the two strands forming a duplex.1 The three methoxy substituents interact with the 2’-methylene group of the nucleoside in the target strand (Figure 3), as shown in a recent high resolution structure.4 Together with the stacking on the terminal base pair, this leads to much-improved mismatch discrimination. The effect is observed for any of the four possible base pairs at the terminus.1
When employed for hybridization probes immobilized on a glass surface in the form of a DNA microarray, the trimethoxystilbene cap increases the signal for the fully complementary target strand.1 This feature is particularly important for A/T-rich sequences that often cause false negatives. The selective stabilization of neighboring Watson-Crick base pairs helps to suppress cross hybridization that would otherwise lead to stronger false positive results.1
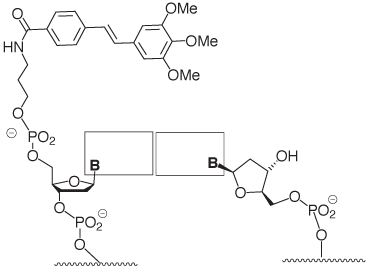
Figure 4 shows a recent example of a hybridization result involving a DNA microarray, where enhanced target signal as well as improved mismatch discrimination at the very terminus of the probe:target duplex are demonstrated.

Results of hybridization experiment with 14mer DNA probes immobilized5 on a DNA microarray and target strand 5'-Cy3-ATCGCAGTCAACCA-3' (incubation at 70 °C for 45min, SSC buffer, pH 7.0; fluorescence readout). The fluorescence scan is shown on the right and the integration on the left. The top row features probes with 5'-attached trimethoxystilbene caps, the bottom row the same probes lacking the caps. Spots in one row differ by the 5'-terminal nucleobase. Capture efficiency and mismatch discrimination are significantly improved in the presence of the cap.
Pyrenylmethylpyrrolindol
This phosphoramidite (2), when employed in the last step of an oligonucleotide synthesis, will produce a cap that is more lipophilic than the trimethoxystilbene. The aromatic stacking moiety is linked to the terminus of the DNA through a more rigid, cyclic linker than in the case of (1). This feature may prove advantageous for researchers interested in exploiting the special photophysical properties of the pyrenyl substituent. The pyrrolindol linker is stereoregular, leading to a single product that can be readily purified by HPLC. The pyrenyl cap is the lead compound discovered in a recent combinatorial study that evaluated over 40 different caps.2 The cap proved particularly successful for hybridization probes with a 5’-terminal deoxyadenosine residue.2 Again, its duplex-stabilizing effect does not require blunt ends. The tertiary amino group can be expected to be protonated at physiological pH, producing a cationic functionality that may help to attract target strands electrostatically. The five membered ring presenting the pyrenyl stacking unit mimics the deoxyribose of natural nucleosides, making duplexes terminating in this cap more similar in shape to unmodified DNA than those capped with the trimethoxystilbene.
We are indebted to Professor Clemens Richert, Institute of Organic Chemistry, University of Karlsruhe, for sharing with us the information included in this article.
References
- Dogan, Z.; Paulini, R.; Rojas Stütz, J. A.; Narayanan, S.; Richert, C. 5’-Tethered stilbene derivatives as fidelity- and affinity-enhancing modulators of DNA duplex stability. J. Am. Chem. Soc. 2004, 126, 4762-4763.
- Narayanan, S.; Gall, J.; Richert, C. Clamping down on weak terminal base pairs: oligonucleotides with molecular caps as fidelity-enhancing elements at the 5’- and 3’-terminal residues. Nucleic Acids Res. 2004, 32, 2901-2911.
-
- Wu, T.; Burch, E. L.; Bassani, D. M.; Yang, J.-S.; Schneider, S.; Jäger, W.; Letsinger, R. L. Hybrid oligonucleotides containing sStilbene units. Excimer fluorescence and photodimerization J. Am. Chem. Soc. 1995, 117, 8785-8792.
- Lewis, F.D.; Wu, T.; Zhang, Y.; Letsinger, R.L., Greenfield, S.R.; Wasielewski, M.R. Distance-dependent electron transfer in DNA hairpins. Science 1997, 277, 673-676.
- Lewis, F. D.; Liu, X.; Wu, Y.; Miller, S. E.; Wasielewski, M. R.; Letsinger, R. L.; Sanishivili, R.; Joachimiak, A.; Tereshko, V.; Egli, M. Structure and photoinduced electron transfer in exceptionally stable synthetic DNA hairpins with stilbenediether linkers. J. Am. Chem. Soc. 1999, 121, 9905-9906.
- Lewis, F. D.; Wu, Y.; Liu, X. Synthesis, structure, and photochemistry of exceptonally stable synthetic DNA hairpins with stilbene diether linkers. J. Am. Chem. Soc. 2002, 124, 12165-12173.
- Tuma, J.; Paulini, R.; Rojas Stütz, J. A.; Richert, C. How much pi-stacking do DNA termini seek? Solution structure of a self-complementary DNA hexamer with trimethoxystilbenes capping the terminal base pairs. Biochemistry 2004, 43, 15680-15687
- Dombi, K. L.; Griesang, N.; Richert, C. Oligonucleotide arrays from aldehyde-bearing glass with coated background. Synthesis 2002, 816-824.
Product Information
5'-Trimethoxystilbene Cap Phosphoramidite (10-1986)
5'-Pyrene Cap Phosphoramidite (10-1987)
- Glen Report 18.11: AP-dC - a Cytosine Analogue Capable of Clamp-like Binding to Guanine - G-clamp
- Glen Report 18.12: Trimer Phosphoramidites – Tools for fine-tuning protein function
- Glen Report 18.13: Sulfurizing Reagent II - Stable in Solution and Optimized for RNA Sulfurization
- Glen Report 18.14: Caps for Increased Duplex Stability and Base-Pairing Fidelity at Termini
- Glen Report 18.15: Synthesis of Branched DNA with a Comb Structure
- Glen Report 18.16: Fluorous Affinity Purification of Oligonucleotides
- Glen Report 18.17: Potent Inhibition of Cytosine-5-methyltransferases
- Glen Report 18-1 Supplement: Sulfurizing Reagent II and its use in Synthesizing Oligonucleotide Phosphorothioates

