Glen Report 18.11: AP-dC - a Cytosine Analogue Capable of Clamp-like Binding to Guanine - G-clamp
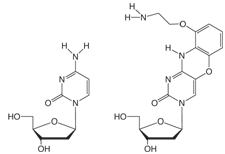
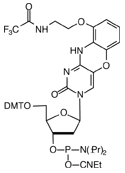
FIGURE 1: dC, AP-dC AND AP-dC-CE PHOSPHORAMIDITE |
|
|
|
 |
dC AP-dC |
AP-dC-CE Phosphoramidite |
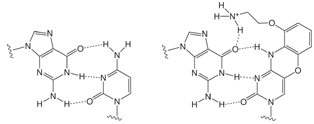
FIGURE 2: G - C AND G - AP-C BASE PAIRING |
|
 |
|
G -------------- C |
G ------------- AP-C |
The ability to modify but, more specifically, to enhance binding between nucleotide bases has always been of critical importance in many avenues of DNA and RNA research. The cytosine – guanine (C-G) base pair with 3 hydrogen bonds is already much stronger than the adenine – thymine (A-T) base pair, which has 2 hydrogen bonds. In this article, we focus on the C-G base pair and a remarkable cytosine analogue, which has been popularly named “G-clamp”.
G-clamp1 is a tricyclic Aminoethyl-Phenoxazine 2’- deoxyCytidine analogue (AP-dC). The nucleoside structure and its relationship to dC are shown in Figure 1, Page 2. The heterocyclic phenoxazine structure provides a stable basis for the protonated amine of an aminoethyl chain to interact with the O6 position of a complementary dG molecule, as shown in Figure 2, Page 2. In principle, therefore, G-clamp should stabilize a duplex due to its ability to interact with both the Watson-Crick and Hoogsteen faces of the target G. In practice, G-clamp did indeed have a very dramatic effect on duplex stabilization. A single G-clamp incorporation in a poly-pyrimidine decamer resulted in a spectacular 18 °C enhancement of the melting temperature of the duplex relative to a control containing 5-Me-dC at the same point.1
Since the G-clamp relies on the hydrogen bonding of its protonated amine to the O6 position of the G target, specificity should, in theory, be enhanced, and indeed it is. The sequence containing a single G-clamp residue exhibited better discrimination between the perfect match with G in comparison to the mismatches with A, C and T.1 In further experiments, it was shown1 that the enhanced binding of G-clamp was not affected by the nature of the bases flanking the target G. It was also shown that the interaction was with G rather than the phosphate backbone when it was determined that the ionic strength of the buffer had little effect on the binding and when uncharged linkages flanked the enhanced base pair.
The specific interaction between G-clamp and the guanine residue should also be unaffected by the nature of the duplex. The melting temperature of a B-form DNA-DNA duplex containing G-clamp was enhanced to about the same extent as an A-form DNA-RNA hybrid.1
Oligonucleotides containing G-clamp have been evaluated2 for sequence-context dependence, activity mismatch, sensitivity, RNAse-H cleavage, and hybridization kinetics in antisense experiments. In previously optimized systems, oligophosphorothioates (S-ON) containing a single G-clamp residue increased the potency of the antisense oligonucleotide even in comparison to the most potent C5-propynyl-modified S-ON previously tested by the researchers.2 Indeed, they conclude that the G-clamp modification is a highly potent, mismatch-sensitive cytosine analog that will find applications in elucidating gene function, in validating gene targets, and in developing more potent antisense oligonucleotides.
Furthermore, a single incorporation of AP-dC at the 3' terminus was shown3 to protect phosphodiester oligonucleotides from 3'-exonuclease digestion.
Our own experiments confirm that AP-dC not only increases hybridization efficiency but, at the same time, the increase is additive over more than one addition of AP-dC. We have also confirmed that AP-dC increases mismatch descrimination. These results are listed in Table 1 and Table 2.
These findings clearly support the conclusion that AP-dC (G-clamp) is a very important addition to the modified nucleosides that enhance hybridization. The product is clearly remarkable for being so stable to conditions of oligonucleotide synthesis and deprotection. We especially envisage its use in the development of short, easily prepared oligonucleotides for use in highly specific single nucleotide polymorphism (SNP) and other in vitro diagnostic assays.
We are indebted to Isis Pharmaceuticals for including AP-dC-CE Phosphoramidite, (1) in Figure 1, to our License Agreement.
References
1. K.Y. Lin and M.D. Matteucci, J Am Chem Soc, 1998, 120, 8531-8532.
2. W.M. Flanagan, et al., Proc Nat Acad Sci USA, 1999, 96, 3513-3518.
3. M.A. Maier, et al., Biochemistry, 2002, 41, 1323-1327.
INTELLECTUAL PROPERTY
This product is covered by patents or patents pending owned by Isis Pharmaceuticals, Inc. (“Isis”). Purchase of this product includes a limited license to use this product solely for internal research. This license specifically excludes (and you have no right to use this product for): (a) therapeutic or diagnostic applications (including products or services that incorporate this product), (b) any in vivo toxicity/safety study in support of an investigational new drug application (or foreign counterpart), (c) resale (including sale of any products or services that incorporate this product) or (d) gene functionalization activities (including products or services that incorporate data derived from gene functionalization activities) if such activities have commercial application, any and all of which require a separate license from Isis. Neither this product nor any product created through its use may be used in human clinical trials.
|
Tm measurements determined by thermal denaturation in a Jasco V530 spectrophotometer. Each oligo was at a concentration of 1 µM in 5mM Tris, 0.1 M NaCl pH 7.4 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tm measurements were carried out as described in Reference 1. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Product Information
AP-dC-CE Phosphoramidite (10-1097)- Glen Report 18.11: AP-dC - a Cytosine Analogue Capable of Clamp-like Binding to Guanine - G-clamp
- Glen Report 18.12: Trimer Phosphoramidites – Tools for fine-tuning protein function
- Glen Report 18.13: Sulfurizing Reagent II - Stable in Solution and Optimized for RNA Sulfurization
- Glen Report 18.14: Caps for Increased Duplex Stability and Base-Pairing Fidelity at Termini
- Glen Report 18.15: Synthesis of Branched DNA with a Comb Structure
- Glen Report 18.16: Fluorous Affinity Purification of Oligonucleotides
- Glen Report 18.17: Potent Inhibition of Cytosine-5-methyltransferases
- Glen Report 18-1 Supplement: Sulfurizing Reagent II and its use in Synthesizing Oligonucleotide Phosphorothioates