Glen Report 25.23: New Product - 5'-Dichloro-Dimethoxy-Fluorescein Phosphoramidite
5'-Dichloro-dimethoxy-fluorescein is more commonly known as JOE™ to those familiar with the dye sets used in Applied Biosystems DNA PRISM sequencers - e.g., Dye Set 20 and 32, which contain FAM/JOE/TAMRA/ROX and FAM/JOE/NED/ROX, respectively.
As a dye, 5'-dichloro-dimethoxy-fluorescein gained popularity because its emission is nicely resolved from both FAM and TAMRA, falling exactly between them. This allows multiplex detection without too much signal bleed through into other channels, making it extremely useful in automated DNA sequencing. In addition, because of the electron-withdrawing groups on the xanthene ring, the 5'-dichloro-dimethoxy-fluorescein dye is less prone to quenching due to protonation. As such, its fluorescence is much less pH sensitive than its popular cousin, fluorescein. Even at pH 5, our in-house testing indicates 5'-dichloro-dimethoxy-fluorescein's signal dropped by only 30%.
A high extinction coefficient of 75,000 L/mol.cm, a quantum yield of fluorescence of 0.58, and excellent stability to standard deprotection conditions in ammonium hydroxide are the reasons why 5'-dichloro-dimethoxy-fluorescein has been a popular addition to our ever-expanding selection of fluorophore phosphoramidites.
This product, (1) in Figure 1, has been available from Glen Research since 2008. Beginning in 2012, due to its increasing popularity, we have been evaluating options for scale up of the current product. Due to several factors, we have concluded that synthesis of this product on a larger scale is not feasible and we have decided to replace it with the optimized product described below.
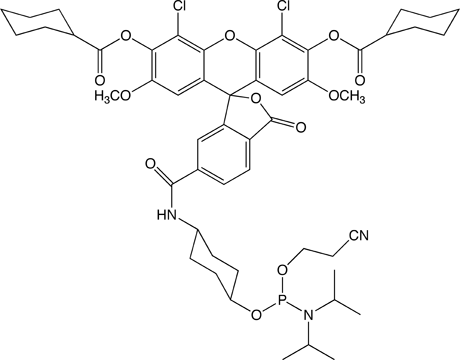
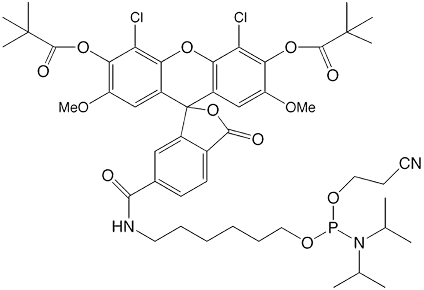
5'-dichloro-dimethoxy-fluorescein II
This improved version of 5'-dichloro-dimethoxy-fluorescein phosphoramidite (2) is shown in Figure 1. This second generation version uses the identical chromophore for 5'-dichloro-dimethoxy-fluorescein but more standard pivaloyl protecting groups are used to protect the fluorophore during synthesis. The spacer used is also the more standard C6 spacer.
Having implemented these changes, we feel confident that we are in a much better position now to support future growth of 5'-dichloro-dimethoxy-fluorescein with much better synthesis scale and consequent pricing profile.
Use of 5'-dichloro-dimethoxy-fluorescein II
- Diluent: Anhydrous Acetonitrile
- Coupling: 6 minute coupling time recommended.
- Deprotection: Use Ammonium Hydroxide and deprotect as required by nucleobases. If AMA is used, a small amount of a non-fluorescent impurity will be formed. To eliminate this impurity, first deprotect with ammonium hydroxide for 30 minutes at room temperature, add an equal volume of 40% methylamine and then complete the deprotection as required by the nucleobases - e.g., 10 minutes at 65°C or 2 hours at room temperature for standard bases.
- Storage: Freezer storage, -10 to -30°C, dry
- Stability in Solution: 1-2 days
It is important to note that the spectral properties of the 5'-Dichloro-dimethoxy-fluorescein fluorophore are unchanged from the original to the new phosphoramidite versions of JOE. Also, the protecting groups and spacer used for the second generation product are actually standard for all of our fluorescein-based products.
Spectral Characteristics
The UV/visible spctrum of a simple T6 oligo labelled with JOE II is shown below:
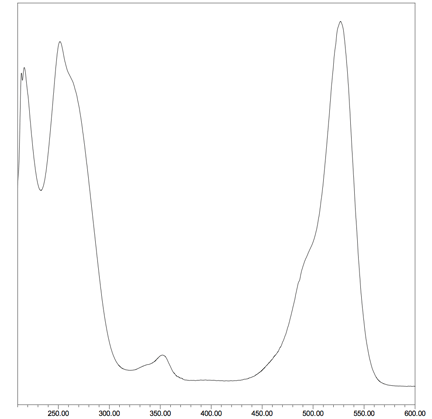
| E260 | 12,000 L/mol.cm |
|---|---|
| Abs. Max. | 525 nm |
| Emax | 75,000 L/mol.cm |
| Emission Max. | 548 nm |
| QY | 0.58 |
Ordering Information
5'-Dichloro-dimethoxy-Fluorescein Phosphoramidite II (Joe II) (10-5906)
- Glen Report 25.21: Methylene Blue Phosphoramidite for DNA Labelling
- Glen Report 25.22: New Product - Methylene Blue C3 Phosphoramidite
- Glen Report 25.23: New Product - 5'-Dichloro-Dimethoxy-Fluorescein Phosphoramidite
- Glen Report 25.24: New Products - TEMPO Spin Labels for Click Chemistry and 5-Ethynyl-dU
- Glen Report 25.25: New Product - 5-Formyl-dC III and the Synthesis of Oligonucleotides Containing All Four Epigenetic Nucleosides
- Glen Report 25.26: Cyanine Dyes - A Personal Perspective
- Glen Report 25.27: Technical Brief - Selective Covalent Capture of DNA and RNA Targets with Shielded Covalent Probes Incorporating a Photo-Activated Crosslinker
- Glen Report 25.28: Technical Brief - Glen UnySupport Now Available With Fast Cleavage

