Glen Report 24.18: Technical Brief – Compatibility of UltraMild Monomers or Ac-dC with Hydrazine Reagent
UltraMild Phosphoramidites (Pac-dA, iPr-Pac-dG, Ac-dC) are versatile phosphoramidites commonly used for the incorporation of modifications that are sensitive to standard deprotection conditions. These phosphoramidites are compatible with a range of deprotection conditions. For UltraMild deprotection we recommend room temperature deprotection with ammonium hydroxide or potassium carbonate in methanol.
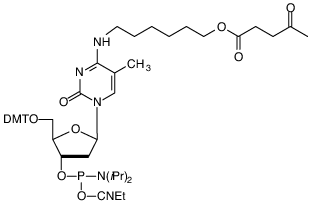
(1) 5-Me-dC Brancher
Acetyl-dC is also a popular monomer in its own right since it is compatible with UltraMild deprotection, as well as regular deprotection using ammonium hydroxide, and is necessary for AMA deprotection.
See our overview of deprotection schemes for further information: Deprotection - Volumes 1-5
Glen Research offers one of the largest selections of phosphoramidites for DNA and RNA synthesis. A popular product is our 5-Me-dC Brancher Phosphoramidite (10-1018; (1)), which is used for synthesizing branched DNA or comb-like DNA structures.1,2 This sequence modifier incorporates a levulinyl-protected alcohol that can serve as a branch point for a second sequence. The process for generating these structures starts by synthesizing the first sequence that incorporates the dC-brancher. After the full-length oligo is synthesized, the DMT is removed and the 5'-hydroxyl is capped with standard Cap A/B reagents. The levulinyl protecting group is then selectively removed with hydrazine hydrate in pyridine/acetic acid solution. The second sequence is then synthesized from the dC brancher.
Recently, we received feedback from our customers that seemed to indicate an incompatibility between the UltraMild phosphoramidites or Ac-dC alone and the protocol for removing the levulinyl protecting group. We suspected the protecting groups of these monomers were prematurely removed during the hydrazine treatment of the dC Brancher, resulting in unwanted branching. The branched sequences were observed as smears on the subsequent PAGE analysis of the final products.
To investigate if the problems were caused by deprotection of the base protecting groups by the hydrazine reagent, we analyzed the stability of Ac-dC to the recommended procedure for removing the levulinyl protecting group. We duly found that the hydrazine reagent can partially deprotect the Ac-dC, leading to chain branching in subsequent coupling cycles.
We conclude that the standard phosphoramidites (Bz-dA, ibu-dG, Bz-dC, and T) should be used when making a branched oligo with our 5-Me-dC Brancher.
Experiment
- Synthesize a control T12 oligo DMT-Off (with no dC incorporations).
- Synthesize two 12-mer T oligos DMT-Off, one with a single internal incorporation of Ac-dC and the other containing Bz-dC.
- Cap the 5'-OH with standard Cap A/B mix.
- Treat oligos with 0.5M hydrazine hydrate in pyridine/acetic acid (1:1) for 15 minutes at room temperature.
- Rinse with pyridine/acetic acid followed by acetonitrile.
- Continue syntheses, adding T6, DMT-ON.
- Deprotect oligos with ammonium hydroxide.
- Analyze on RP-HPLC.
Results
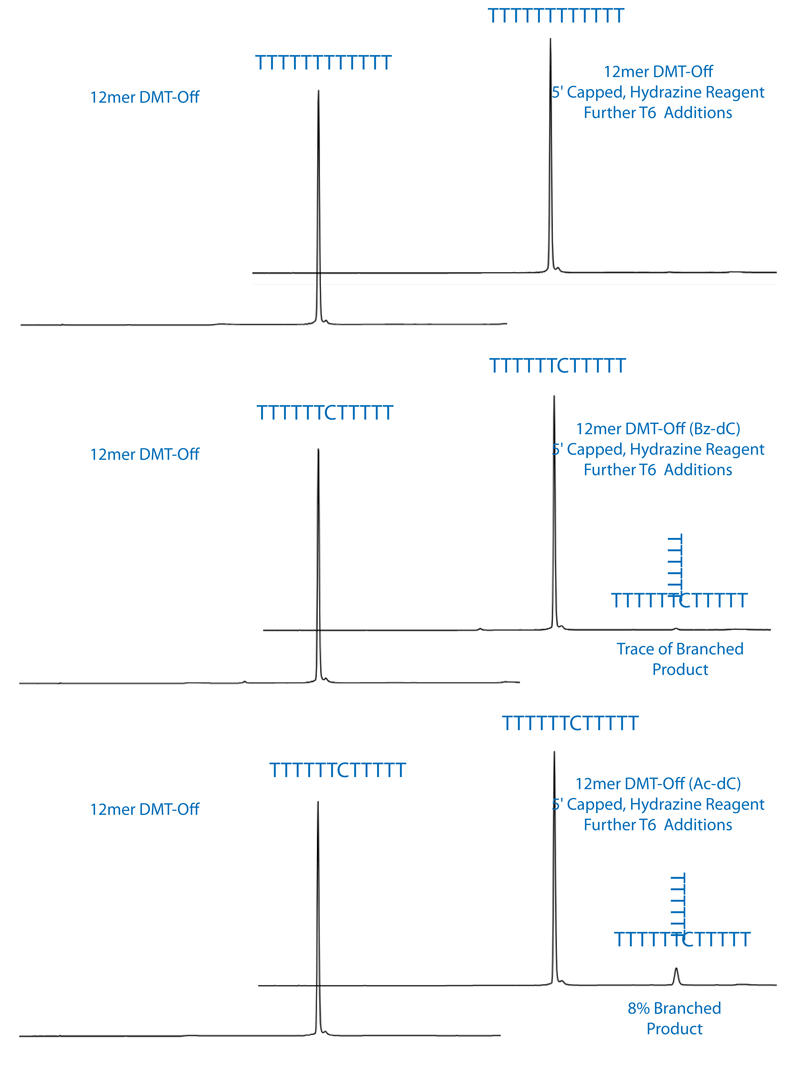
Branched oligos would contain a 5’-DMT group and have a significantly longer retention time on RP-HPLC. The control T12 did not show any signs of branching as seen by the absence of any DMT containing oligo. This also indicates that the 5’-acetyl capping group was retained during the hydrazine hydrate deprotection. The Ac-dC containing oligo did have a late eluting peak on RP-HPLC that would be consistent with branching. We calculated about 8% branching after a 15 minute treatment with hydrazine hydrate. The Bz-dC containing oligo showed only a trace amount of branched oligo. The chromatograms are shown in Figure 2.
References:
- T. Horn, C.A. Chang, and M.S. Urdea, Nucleic Acids Res, 1997, 25, 4842-4849.
- T. Horn, C.A. Chang, and M.S. Urdea, Nucleic Acids Res, 1997, 25, 4835-4841.
Product Information
- Glen Report 24.11: Spermine Phosphoramidite: A Potent Modification with Many Applications
- Glen Report 24.12: New Product - S-Bz-Thiol-Modifier C6-dT
- Glen Report 24.13: New Products: AB 3900 Columns Containing 1000Å CPG
- Glen Report 24.14: New Product - Dibenzaocyclooctyl (DBCO) Copper-Free Click Chemistry
- Glen Report 24.15: New Products - 6-HEX and 6-TET Azides
- Glen Report 24.16: New Products - Solid, Stable Amino-Modifiers - PDA Amino-Modifiers
- Glen Report 24.17: New Product - 5'-Stearyl Phosphoramidite - An Alternative Lipophilic Carrier
- Glen Report 24.18: Technical Brief – Compatibility of UltraMild Monomers or Ac-dC with Hydrazine Reagent

