The copper(I) catalyzed [3+2] azide-alkyne cycloaddition (CuAAC) is the most prominent example of a group of reactions known as click reactions, as shown in Figure 1. This cycloaddition reaction is characterized by its high efficency, mild reaction conditions, and by its tolerance of the broad range of functional groups found in DNA.1 The most important characteristic of the CuAAC reaction is its unique bioorthogonality, as neither azide nor terminal alkyne functional groups are generally present in natural systems. Despite the attractive features of CuAAC-based click chemistry, the presence of copper may be a hindrance to bioorthogonal conjugation in living cells or whole organisms.

Recently, developers of bioorthogonal techniques have begun to consider the use of highly strained alkynes to obviate the need for copper catalysis in click conjugation reactions. Cyclooctyne is the smallest cyclic octyne that can be isolated. Because of the severe deformation of the alkyne from its desired linear geometry, cyclooctynes are highly reactive towards azides without the need for copper catalysis.
At Glen Research, our goal was to offer a copper-free click phosphoramidite reagent with the following properties:
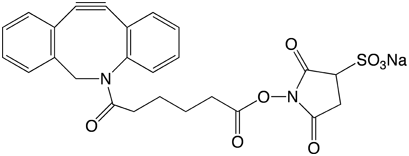
From the variety of cyclooctyne-based copper-free click reagents so far described, we have chosen to offer compounds based on a dibenzo-bicyclo-octyne (DBCO) structure, shown in Figure 2.2-4 Further, we chose to separate this very hydrophobic moiety from the phosphoramidite and subsequent oligo with a triethyleneglycol (TEG) spacer. The structure of the 5’-DBCO-TEG Phosphoramidite (1) is shown in Figure 3. In addition, we have chosen to offer a soluble DBCO-sulfo-NHS ester sodium salt (2) for post-synthesis conjugation reactions with amino-modified oligonucleotides and proteins.


A coupling time of 10 minutes was found to be optimal for the phosphoramidite (1). It was found that DBCO-modified oligos were stable to deprotection with ammonium hydroxide for 2 hours at 65°C or overnight at room temperature, which would allow the use of regular phosphoramidites, including dmf-dG but not ibu-dG. Deprotection with AMA for 2 hours at room temperature showed only slight degradation of the cyclooctyne, making the modification compatible with ibu-dG if Ac-dC is used. DBCO-modified oligos are also compatible with UltraMild deprotection conditions.
5’-DBCO-modified oligos are sufficiently hydrophobic that they may be purified using Glen-Pak™ cartridges and omitting the TFA step normally used to remove the DMT group.
5’-DBCO-modified oligos may be conjugated with azides in organic solvents, such as DMSO, or aqeous buffers. Depending on the azide used, the reaction will go to completion in 4-17 hours at room temperature. Simple desalting on a Glen Gel-Pak™ leads to a product with virtually quantitative conjugation efficiency.
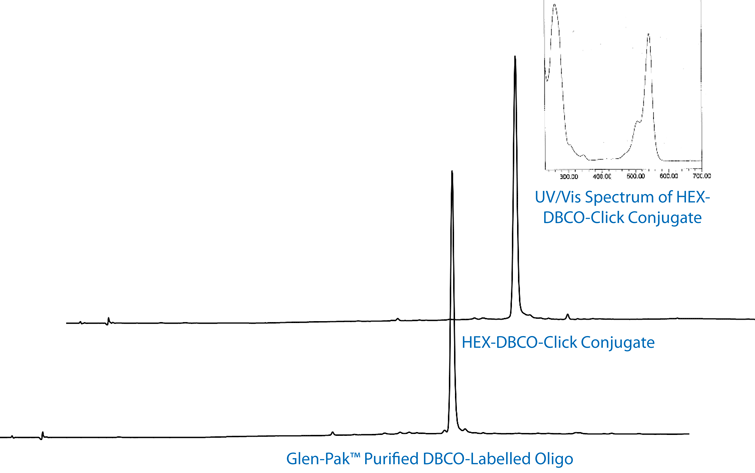
We illustrate the efficiency of the copper-free click reaction using 6-HEX Azide, one of two additions to our selection of azides for click chemistry, in Figure 5.
Dissolve DBCO-sulfo-NHS Ester at a concentration of 5.2mg per 60 µL (~0.17M solution) in water or anhydrous DMSO. Use this stock solution to conjugate with amino-modified oligos in sodium carbonate/bicarbonate conjugation buffer (pH=9).
For a 0.2 µmole synthesis of an amino-modified oligo:
In Figure 5, the high conjugation efficiency of 6-HEX Azide is illustrated. Virtually 100% efficiency was achieved in a copper-free click with an oligo modified at the 5' terminus using 5’-DBCO-TEG Phosphoramidite.
Also see: New Products - 6-HEX and 6-TET Azides
5'-DBCO-TEG Phosphoramidite (10-1941)
DBCO-sulfo-NHS Ester (50-1941)
Glen-Pak™ and Glen Gel-Pak™ are trademarks of Glen Research Corporation.