Glen Report 23.12: New Products Prevent Branching at Secondary Amines using DCI Activator
The Glen Research catalog of products includes several where a secondary amine remains unprotected. However, all of our work with these products indicated that branching during synthesis due to the coupling of phosphoramidites at the secondary amine occurred at a very low level. Indeed, efficient manufacture of the phosphoramidites would not be possible if significant phosphitylation occurred at the secondary amino position. All of that work was carried out using 1H-tetrazole as activator. During subsequent work using 4,5-dicyanoimidazole (DCI) as activator, we noticed in some routine experiments that branching at the secondary amino positions was very significant.
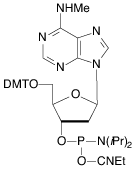
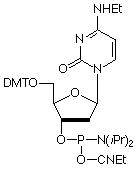
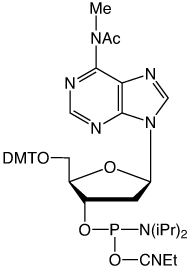
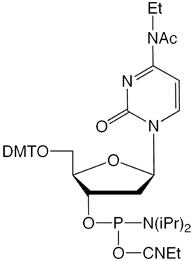
Our main causes for concern were two minor base phosphoramidites, N6-Me-dA (10-1003) (1) and N4-Et-dC (10-1068)1 (2), as well as unprotected biotin phosphoramidites. For example, Figure 2 shows the differences between two simple oligos containing N4-Et-dC when using 1H-tetrazole or DCI as activator. Similarly, Figure 3 illustrates the coupling of N4-Et-dC to a universal support using 1H-tetrazole or DCI. From both experiments, coupling with 1H-tetrazole leads to a trace of branching, while DCI leads to around 15% branching.


Our first priority was to address the situation with these two popular minor base analogues. In collaboration with Berry and Associates, the acetyl protected monomers (3) and (4) were prepared. Acetyl protection was chosen since it would block branching reactions while being compatible with all deprotection strategies from UltraMild to UltraFast.
Oligonucleotides synthesized using monomers (3) and (4) indeed proved to be compatible with all popular deprotection strategies. When the acetyl protected monomers were compared with the unprotected monomers using DCI as activator, branching was reduced from 15% to zero.
We are happy to add the acetyl protected monomers to our catalog but we will also continue to maintain supply of the unprotected monomers for optimal use with 1H-tetrazole or other members of the tetrazole family of activators.
Increased levels of branching are also observed when unprotected biotin products are used along with DCI as activator. In this case, branching can be avoided by using tetrazole activators. Alternatively, we have a line of new serinol-based biotin products that are already protected with the t-butylbenzoyl group. Biotin-dT is similarly protected, although the protecting group in this case was originally chosen to confer better solubility to the product. 5’-Biotin phosphoramidite is already protected with a DMT group. All of our biotin supports are aggressively capped as acetates to include the biotin N1 position so minimal branching should be observed from our supports.
Reference
- H.K. Nguyen, P. Auffray, U. Asseline, D. Dupret, and N.T. Thuong, Nucleic Acids Res, 1997, 25, 3059-65.
Ordering Information
N6-Ac-N6-Me-dA-CE Phosphoramidite (10-1503)
N4-Ac-N4-Et-dC-CE Phosphoramidite (10-1513)
- Glen Report 23.11: DNA Methylation Revisited
- Glen Report 23.12: New Products Prevent Branching at Secondary Amines using DCI Activator
- Glen Report 23.13: New Products – Click Chemistry Update
- Glen Report 23.14: New Product – Ultrafast Photo Cross-Linker
- Glen Report 23.15: Product Update - Unnatural Base Pairs
- Glen Report 23.16: Technical Brief - Labelling Carboxy-Modifiers with Multiple Reporter Molecules
- Glen Report 23.17: Technical Brief - Chemical Phosphorylation – Considering the Options

